Determining p H and titrations Indicators and p







- Slides: 13

Determining p. H and titrations

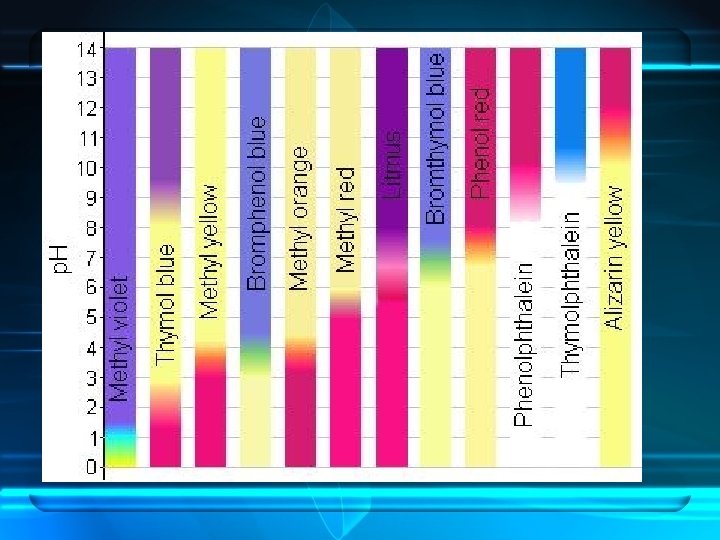
Indicators and p. H meters • You can get an approximate value for p. H by using an acid-base indicator (compounds whose colors are sensitive to p. H) • Indicators come in different colors and have different ranges over which their color changes (transition interval) • See chart on p. 513 for some indicators, their transition intervals, and their color changes


• Universal indicators are made by mixing several different indicators • Paper soaked in universal indicator is called p. H paper • When an exact value for p. H is needed, a p. H meter is used. – Measures the voltage between two electrodes – The voltage changes as the hydronium ion concentration changes

Titration • The controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measured amount of solution of unknown concentration. • For example, if you have a solution which is acidic and you need to know what the molarity is, you can titrate a certain amount of the solution with a basic solution of known molarity

Titration • Equivalence point – the point at which both solutions are present in chemically equivalent amounts • End point – the point at which the indicator changes color.

Titration • Indicators with a small transition interval are the best to use. • When titrating a strong acid with a strong base, the neutralization will produce a salt in water with a p. H of about 7 so an indicator that changes color at a p. H of 7 is needed.

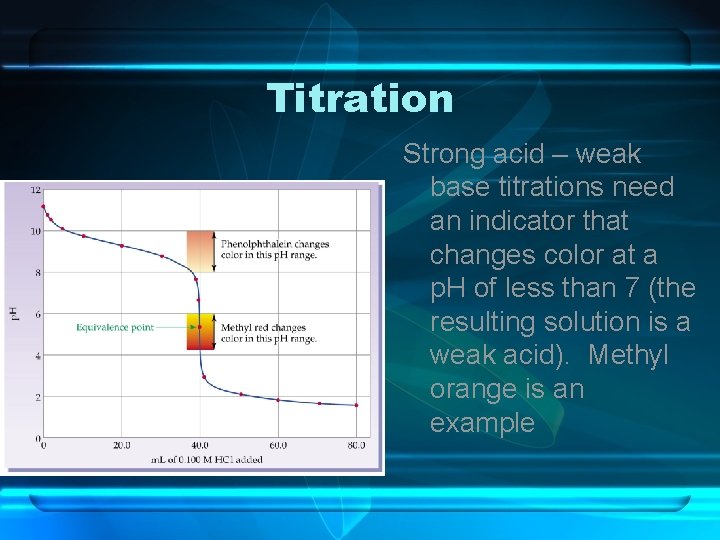
Titration Strong acid – weak base titrations need an indicator that changes color at a p. H of less than 7 (the resulting solution is a weak acid). Methyl orange is an example

Titration Weak acid – strong base titrations require an indicator that changes color at a p. H of more than 7 because the resulting solution is a weak base. Phenolphthalein is an example

Titration • Put a measured amount of acid solution into a flask, add indicator • Put a basic solution of known molarity into the buret • Add the base little by little – the p. H changes slowly at first, then rapidly through the equivalence point, then slowly again as the solution becomes more basic. • Record the amount of base needed to get to the equivalence point • With the amounts of both solutions known and the molarity of the base known, the molarity of the acid can be calculated.

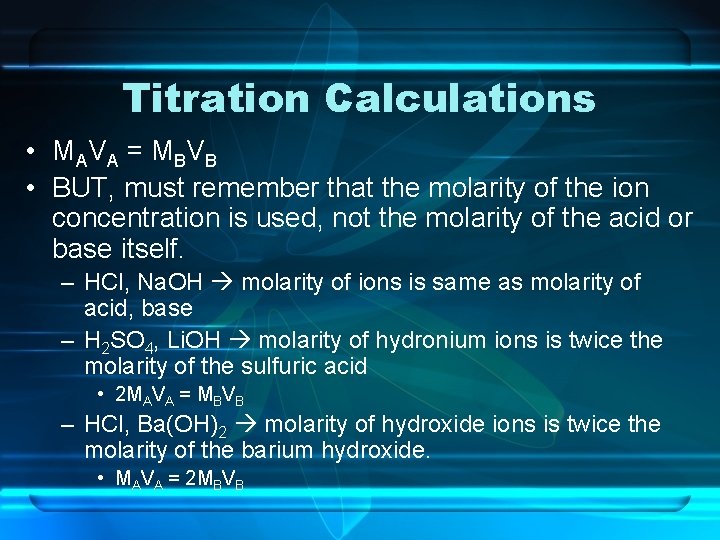
Titration Calculations • MA V A = M B V B • BUT, must remember that the molarity of the ion concentration is used, not the molarity of the acid or base itself. – HCl, Na. OH molarity of ions is same as molarity of acid, base – H 2 SO 4, Li. OH molarity of hydronium ions is twice the molarity of the sulfuric acid • 2 MAVA = MBVB – HCl, Ba(OH)2 molarity of hydroxide ions is twice the molarity of the barium hydroxide. • MAVA = 2 MBVB

Titration Calculations • A 15. 5 m. L sample of 0. 215 M KOH solution required 21. 2 m. L of aqueous acetic acid solution in a titration experiment. Calculate the molarity of the acetic acid solution.

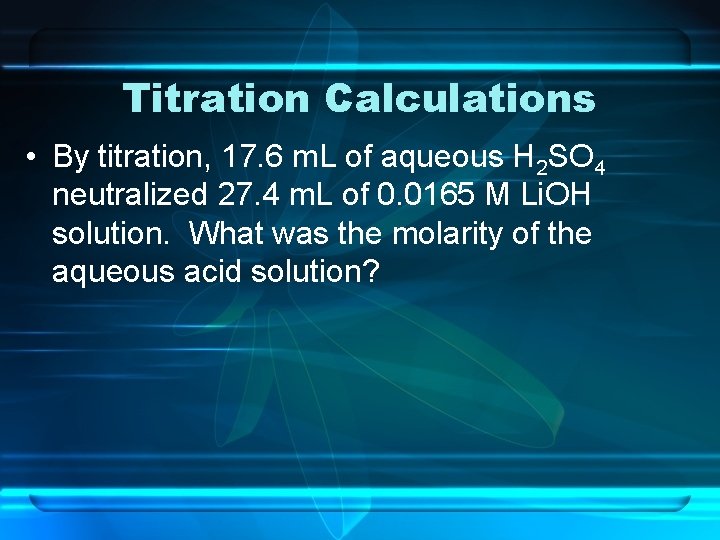
Titration Calculations • By titration, 17. 6 m. L of aqueous H 2 SO 4 neutralized 27. 4 m. L of 0. 0165 M Li. OH solution. What was the molarity of the aqueous acid solution?