Determining Lewis Dot Structures In order to determine
- Slides: 14

Determining Lewis Dot Structures In order to determine the Lewis dot structure for any molecule, follow the following steps: 1. Draw skeletal structure of compound showing what atoms are bonded to each other. Put least electronegative element in the center. 2. Count total number of valence e-. Add 1 for each negative charge. Subtract 1 for each positive charge. 3. Complete an octet for all atoms except hydrogen two, Lithium four, Beryllium 6. 4. lf structure contains too many electrons, form double and triple bonds on central atom as needed.

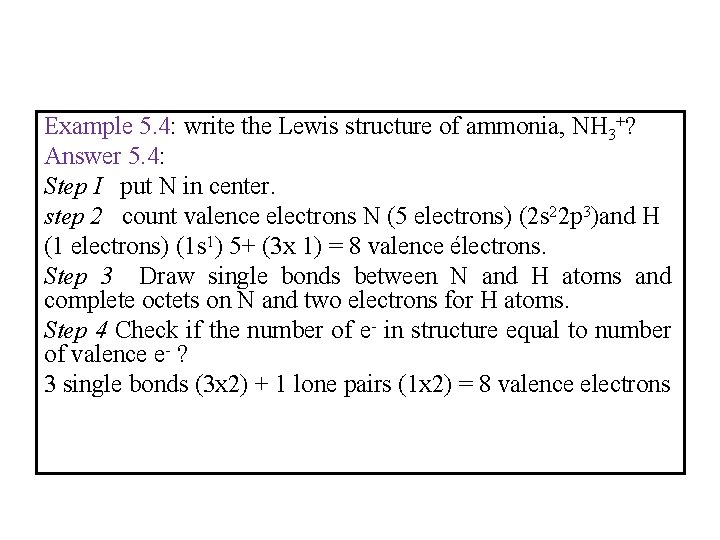
Example 5. 4: write the Lewis structure of ammonia, NH 3+? Answer 5. 4: Step I put N in center. step 2 count valence electrons N (5 electrons) (2 s 22 p 3)and H (1 electrons) (1 s 1) 5+ (3 x 1) = 8 valence électrons. Step 3 Draw single bonds between N and H atoms and complete octets on N and two electrons for H atoms. Step 4 Check if the number of e- in structure equal to number of valence e- ? 3 single bonds (3 x 2) + 1 lone pairs (1 x 2) = 8 valence electrons

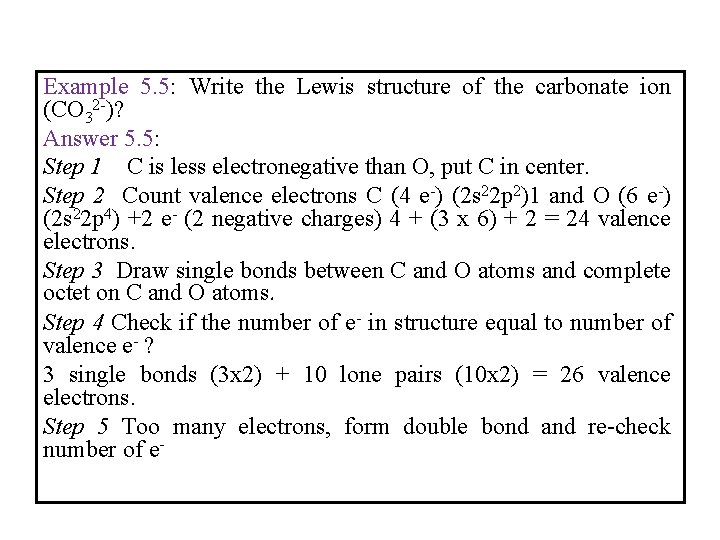
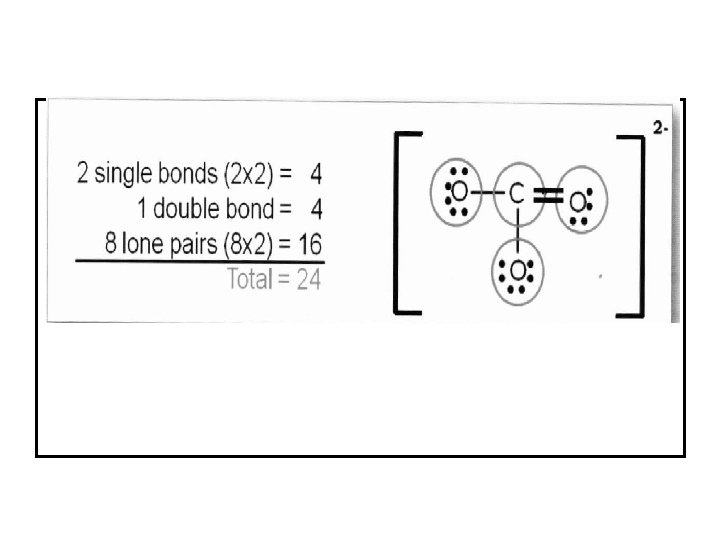
Example 5. 5: Write the Lewis structure of the carbonate ion (CO 32 -)? Answer 5. 5: Step 1 C is less electronegative than O, put C in center. Step 2 Count valence electrons C (4 e-) (2 s 22 p 2)1 and O (6 e-) (2 s 22 p 4) +2 e- (2 negative charges) 4 + (3 x 6) + 2 = 24 valence electrons. Step 3 Draw single bonds between C and O atoms and complete octet on C and O atoms. Step 4 Check if the number of e- in structure equal to number of valence e- ? 3 single bonds (3 x 2) + 10 lone pairs (10 x 2) = 26 valence electrons. Step 5 Too many electrons, form double bond and re-check number of e-


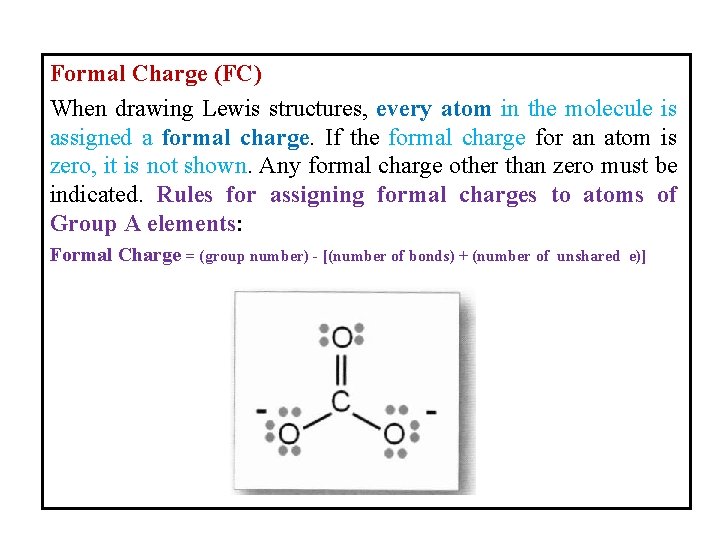
Formal Charge (FC) When drawing Lewis structures, every atom in the molecule is assigned a formal charge. If the formal charge for an atom is zero, it is not shown. Any formal charge other than zero must be indicated. Rules for assigning formal charges to atoms of Group A elements: Formal Charge = (group number) - [(number of bonds) + (number of unshared e)]

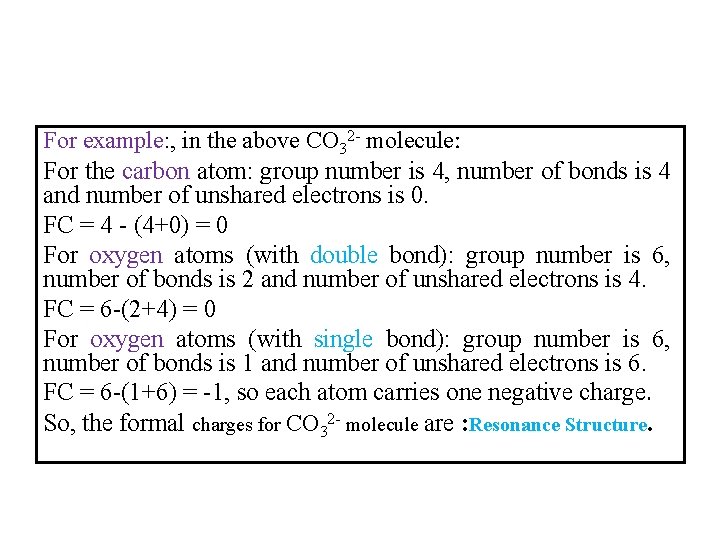
For example: , in the above CO 32 - molecule: For the carbon atom: group number is 4, number of bonds is 4 and number of unshared electrons is 0. FC = 4 - (4+0) = 0 For oxygen atoms (with double bond): group number is 6, number of bonds is 2 and number of unshared electrons is 4. FC = 6 -(2+4) = 0 For oxygen atoms (with single bond): group number is 6, number of bonds is 1 and number of unshared electrons is 6. FC = 6 -(1+6) = -1, so each atom carries one negative charge. So, the formal charges for CO 32 - molecule are : Resonance Structure.

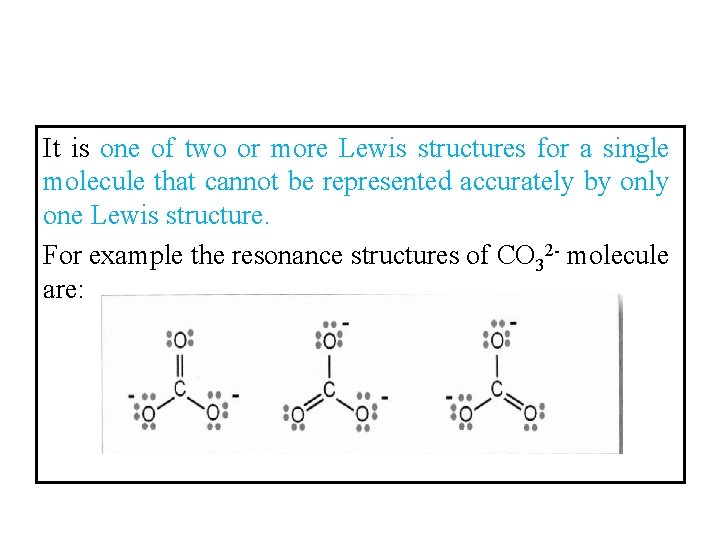
It is one of two or more Lewis structures for a single molecule that cannot be represented accurately by only one Lewis structure. For example the resonance structures of CO 32 - molecule are:

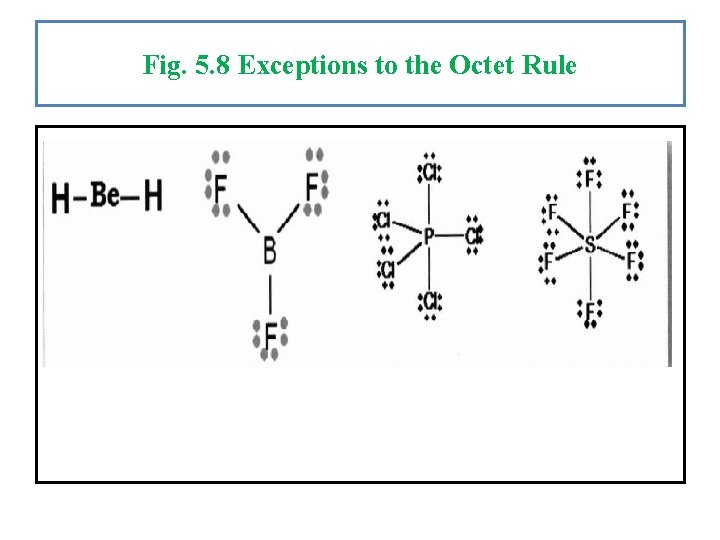
Exceptions to the Octet Rule: Most of the atoms in the periodic table follow the octet rule, but there are some exceptions to the octet rule: 1) Fewer than 8 valence electron (incomplete octet): Also, Group 2 A elements, such as beryllium, have only 2 valence electrons available for sharing. So, the maximum number of electrons can be present around Be is 4 (two bonds). Group 3 A elements, such as boron, has only 3 valence electrons available for sharing. So, the maximum number of electrons can be present around B is 6 (three bonds). 2) More than 8 valence electrons (expanded octet): In periods 3 and beyond , d-orbitals can be used in bonding, which allows an atom to have an expanded octet (more than 8 electrons). For example: Sulfur in SF 6 is surrounded by 12 electrons and phosphorus in PCl 5 is surrounded by 10 electrons.

Fig. 5. 8 Exceptions to the Octet Rule

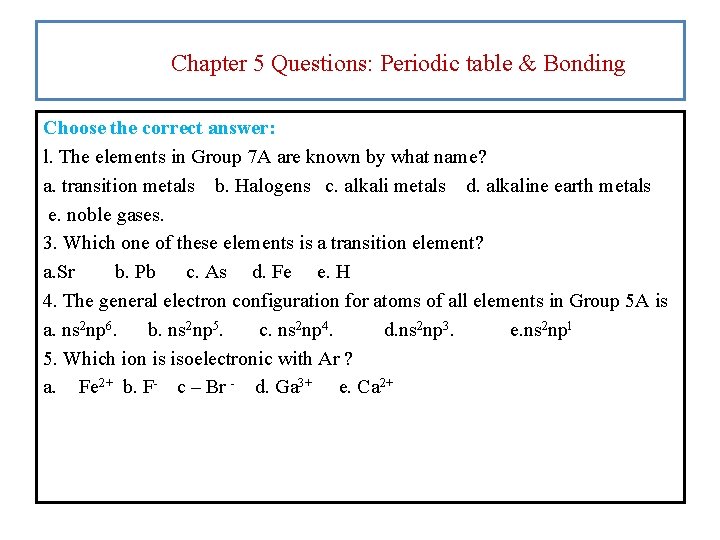
Chapter 5 Questions: Periodic table & Bonding Choose the correct answer: l. The elements in Group 7 A are known by what name? a. transition metals b. Halogens c. alkali metals d. alkaline earth metals e. noble gases. 3. Which one of these elements is a transition element? a. Sr b. Pb c. As d. Fe e. H 4. The general electron configuration for atoms of all elements in Group 5 A is a. ns 2 np 6. b. ns 2 np 5. c. ns 2 np 4. d. ns 2 np 3. e. ns 2 npl 5. Which ion is isoelectronic with Ar ? a. Fe 2+ b. F- c – Br - d. Ga 3+ e. Ca 2+

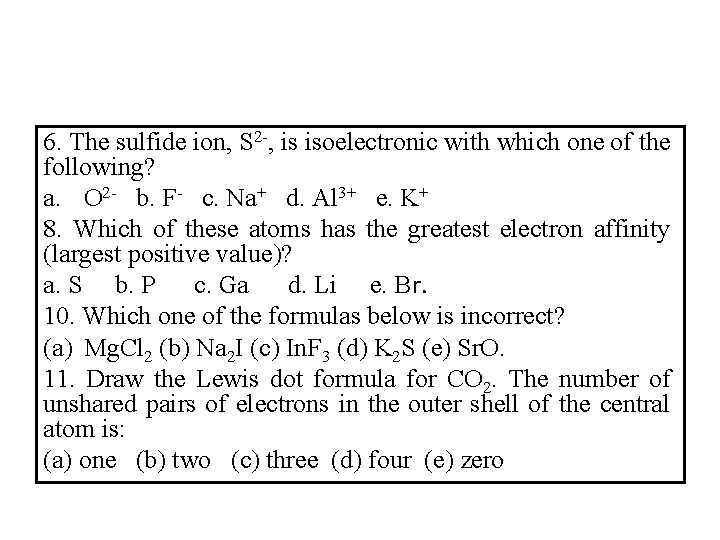
6. The sulfide ion, S 2 -, is isoelectronic with which one of the following? a. O 2 - b. F- c. Na+ d. Al 3+ e. K+ 8. Which of these atoms has the greatest electron affinity (largest positive value)? a. S b. P c. Ga d. Li e. Br. 10. Which one of the formulas below is incorrect? (a) Mg. Cl 2 (b) Na 2 I (c) In. F 3 (d) K 2 S (e) Sr. O. 11. Draw the Lewis dot formula for CO 2. The number of unshared pairs of electrons in the outer shell of the central atom is: (a) one (b) two (c) three (d) four (e) zero

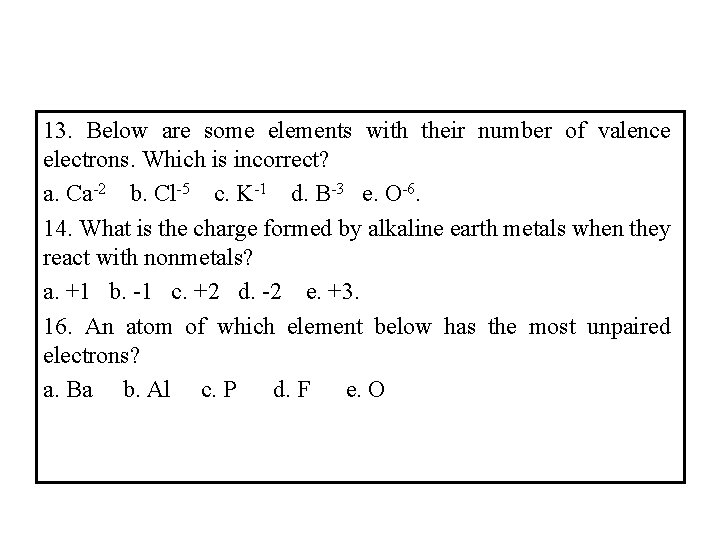
13. Below are some elements with their number of valence electrons. Which is incorrect? a. Ca-2 b. Cl-5 c. K-1 d. B-3 e. O-6. 14. What is the charge formed by alkaline earth metals when they react with nonmetals? a. +1 b. -1 c. +2 d. -2 e. +3. 16. An atom of which element below has the most unpaired electrons? a. Ba b. Al c. P d. F e. O

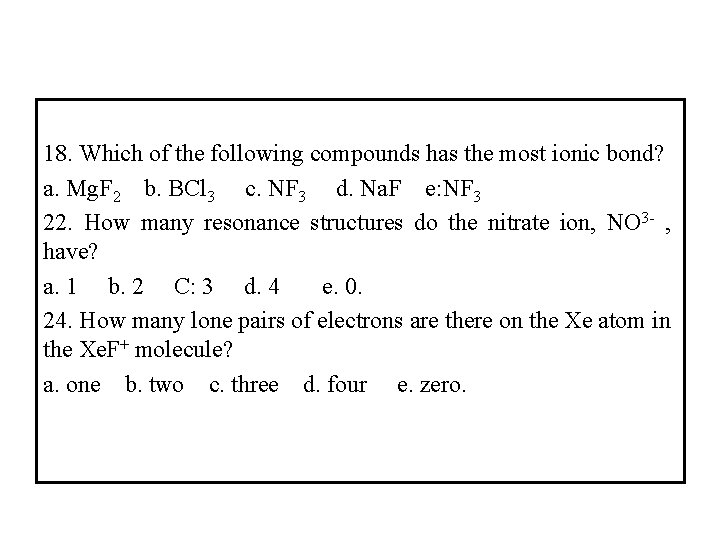
18. Which of the following compounds has the most ionic bond? a. Mg. F 2 b. BCl 3 c. NF 3 d. Na. F e: NF 3 22. How many resonance structures do the nitrate ion, NO 3 - , have? a. 1 b. 2 C: 3 d. 4 e. 0. 24. How many lone pairs of electrons are there on the Xe atom in the Xe. F+ molecule? a. one b. two c. three d. four e. zero.

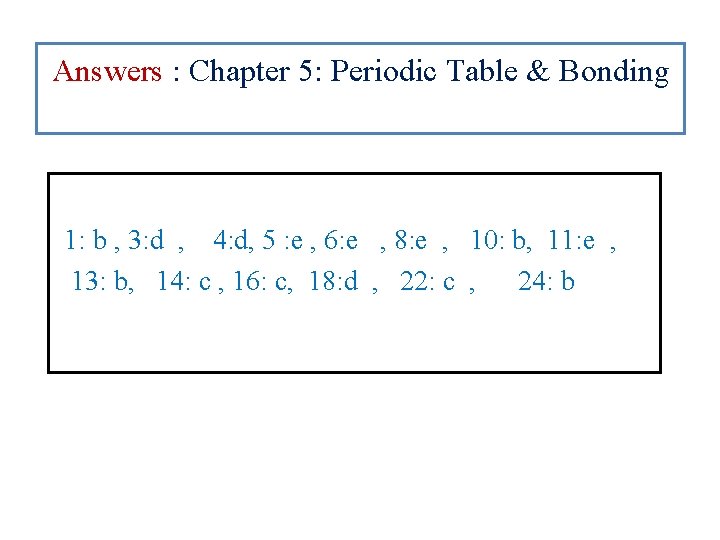
Answers : Chapter 5: Periodic Table & Bonding 1: b , 3: d , 4: d, 5 : e , 6: e , 8: e , 10: b, 11: e , 13: b, 14: c , 16: c, 18: d , 22: c , 24: b