Determining formulae The percentage composition of a compound




- Slides: 36

Determining formulae The percentage composition of a compound leads directly to its empirical formula. Recall: An empirical formula for a compound is the formula of a substance written with the smallest integer subscripts. Eg. Consider hydrogen peroxide: Molecular formula = H 2 O 2 Empirical formula = HO Timberlake Lecture. PLUS 1

Types of Formulas The formulas for compounds can be expressed as an empirical formula and as a molecular(true) formula. Empirical Molecular (true) Name CH C 2 H 2 acetylene CH C 6 H 6 benzene CO 2 CH 2 O C 5 H 10 O 5 Timberlake Lecture. PLUS carbon dioxide ribose 2

Compounds with different molecular formulae can have the same empirical formula, and such substances will have the same percentage composition. Eg. acetylene = C 2 H 2 benzene = C 6 H 6 both have the empirical formula = ? Timberlake Lecture. PLUS 3

Empirical Formulas Write your own one-sentence definition for each of the following: Empirical formula Molecular formula Timberlake Lecture. PLUS 4

• An empirical formula represents the simplest whole number ratio of the atoms in a compound. • The molecular formula is the true or actual ratio of the atoms in a compound. Timberlake Lecture. PLUS 5

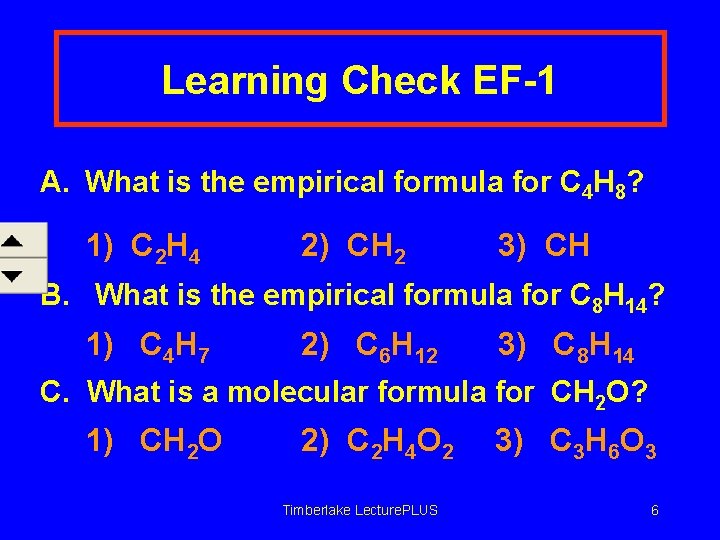
Learning Check EF-1 A. What is the empirical formula for C 4 H 8? 1) C 2 H 4 2) CH 2 3) CH B. What is the empirical formula for C 8 H 14? 1) C 4 H 7 2) C 6 H 12 3) C 8 H 14 C. What is a molecular formula for CH 2 O? 1) CH 2 O 2) C 2 H 4 O 2 Timberlake Lecture. PLUS 3) C 3 H 6 O 3 6

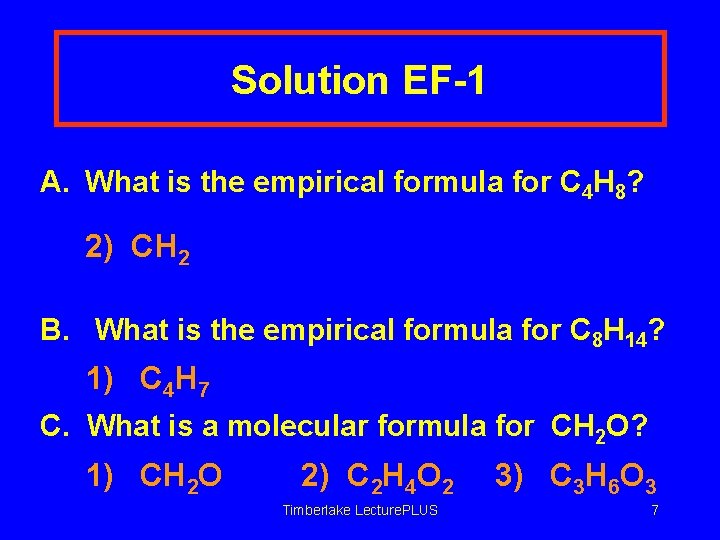
Solution EF-1 A. What is the empirical formula for C 4 H 8? 2) CH 2 B. What is the empirical formula for C 8 H 14? 1) C 4 H 7 C. What is a molecular formula for CH 2 O? 1) CH 2 O 2) C 2 H 4 O 2 Timberlake Lecture. PLUS 3) C 3 H 6 O 3 7

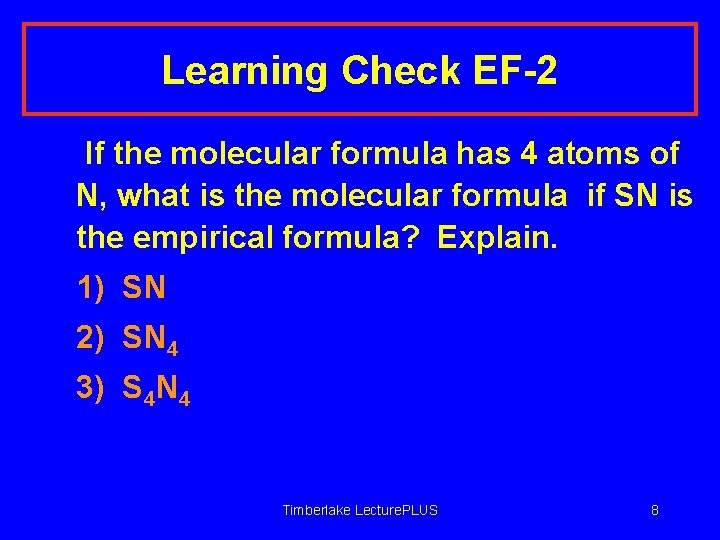
Learning Check EF-2 If the molecular formula has 4 atoms of N, what is the molecular formula if SN is the empirical formula? Explain. 1) SN 2) SN 4 3) S 4 N 4 Timberlake Lecture. PLUS 8

Solution EF-2 If the molecular formula has 4 atoms of N, what is the molecular formula if SN is the empirical formula? Explain. 3) S 4 N 4 If the actual formula has 4 atoms of N, and S is related 1: 1, then there must also be 4 atoms of S. Timberlake Lecture. PLUS 9

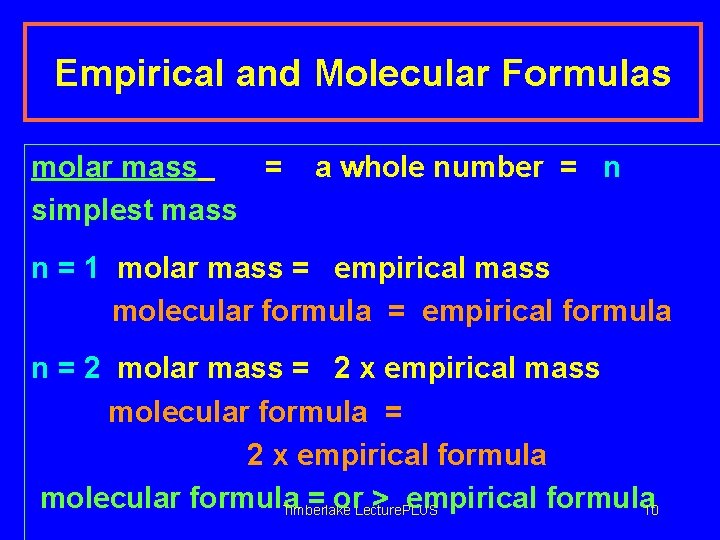
Empirical and Molecular Formulas molar mass = simplest mass a whole number = n n = 1 molar mass = empirical mass molecular formula = empirical formula n = 2 molar mass = 2 x empirical mass molecular formula = 2 x empirical formula molecular formula = or > empirical formula 10 Timberlake Lecture. PLUS

Empirical Formula Empirical Mass Timberlake Lecture. PLUS Molecular Formula Molecular Mass 11

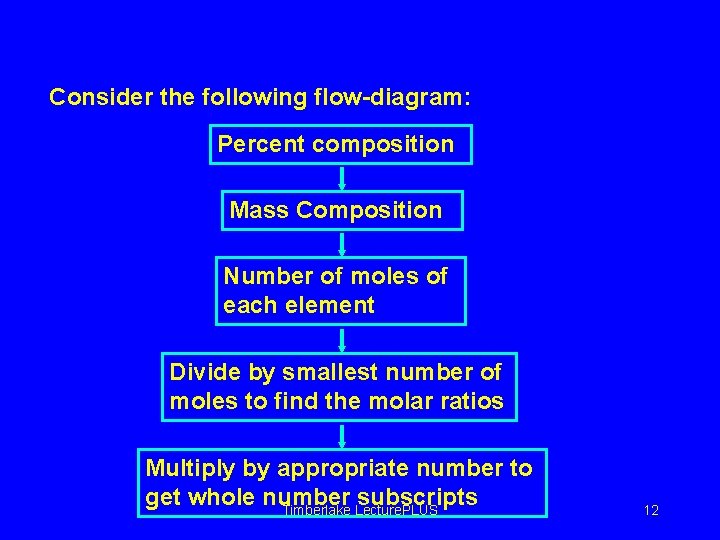
Empirical formula from Composition Consider the following flow-diagram: Percent composition Mass Composition Number of moles of each element Divide by smallest number of moles to find the molar ratios Multiply by appropriate number to get whole number subscripts Timberlake Lecture. PLUS 12

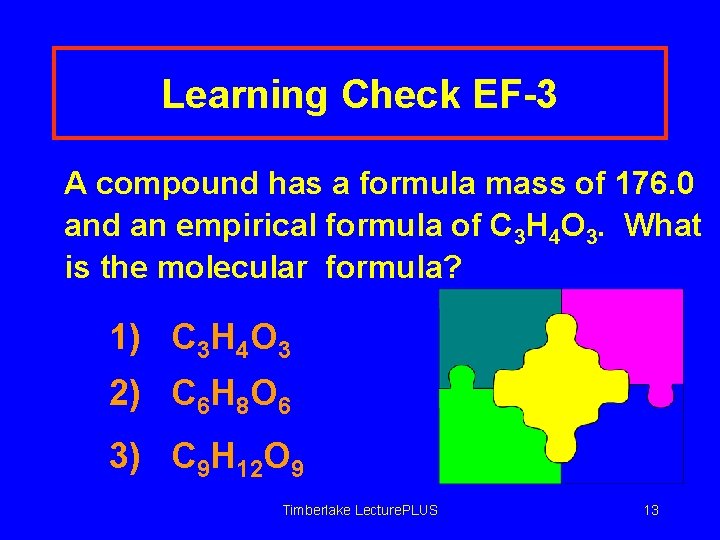
Learning Check EF-3 A compound has a formula mass of 176. 0 and an empirical formula of C 3 H 4 O 3. What is the molecular formula? 1) C 3 H 4 O 3 2) C 6 H 8 O 6 3) C 9 H 12 O 9 Timberlake Lecture. PLUS 13

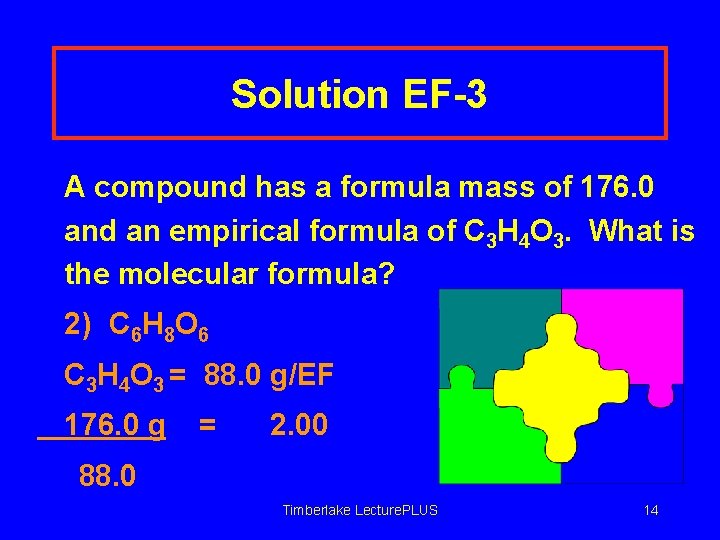
Solution EF-3 A compound has a formula mass of 176. 0 and an empirical formula of C 3 H 4 O 3. What is the molecular formula? 2) C 6 H 8 O 6 C 3 H 4 O 3 = 88. 0 g/EF 176. 0 g = 2. 00 88. 0 Timberlake Lecture. PLUS 14

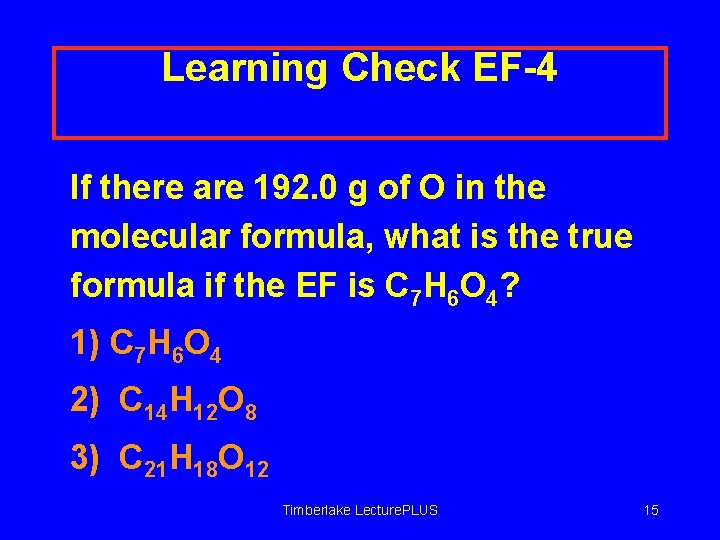
Learning Check EF-4 If there are 192. 0 g of O in the molecular formula, what is the true formula if the EF is C 7 H 6 O 4? 1) C 7 H 6 O 4 2) C 14 H 12 O 8 3) C 21 H 18 O 12 Timberlake Lecture. PLUS 15

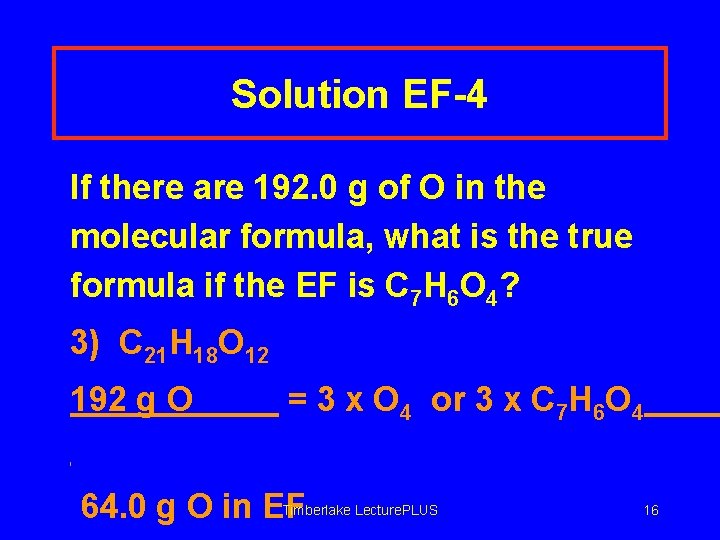
Solution EF-4 If there are 192. 0 g of O in the molecular formula, what is the true formula if the EF is C 7 H 6 O 4? 3) C 21 H 18 O 12 192 g O = 3 x O 4 or 3 x C 7 H 6 O 4 Timberlake Lecture. PLUS 64. 0 g O in EF 16

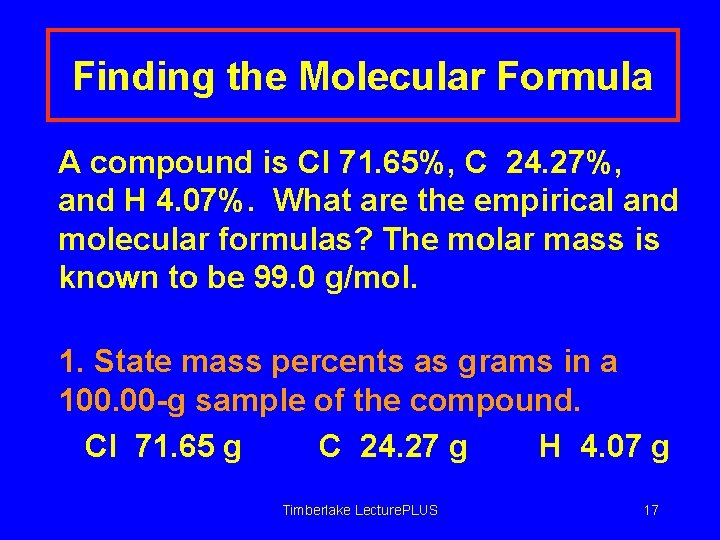
Finding the Molecular Formula A compound is Cl 71. 65%, C 24. 27%, and H 4. 07%. What are the empirical and molecular formulas? The molar mass is known to be 99. 0 g/mol. 1. State mass percents as grams in a 100. 00 -g sample of the compound. Cl 71. 65 g C 24. 27 g H 4. 07 g Timberlake Lecture. PLUS 17

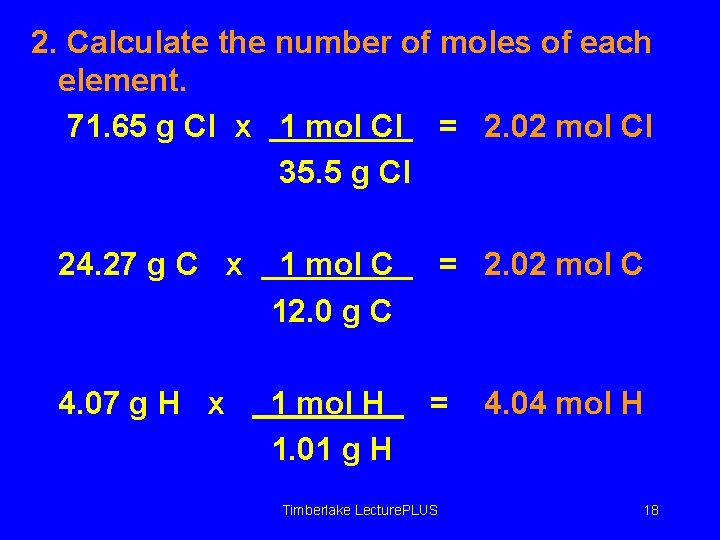
2. Calculate the number of moles of each element. 71. 65 g Cl x 1 mol Cl = 2. 02 mol Cl 35. 5 g Cl 24. 27 g C x 1 mol C 12. 0 g C = 2. 02 mol C 4. 07 g H x 1 mol H 1. 01 g H = Timberlake Lecture. PLUS 4. 04 mol H 18

Why moles? Why do you need the number of moles of each element in the compound? Timberlake Lecture. PLUS 19

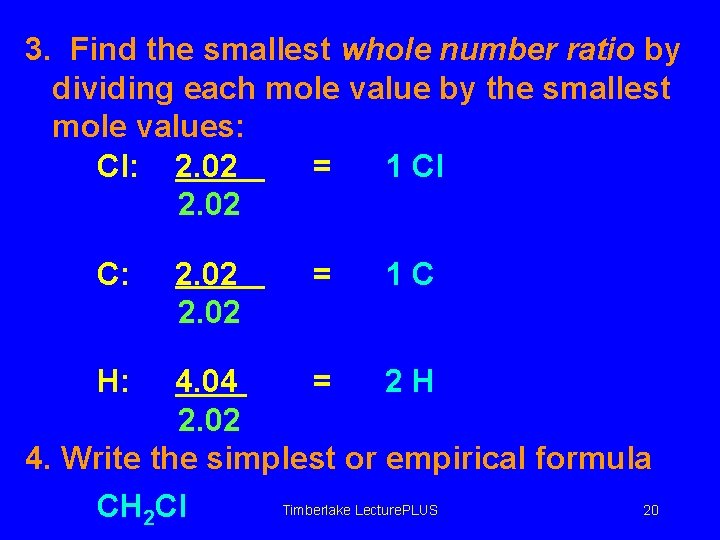
3. Find the smallest whole number ratio by dividing each mole value by the smallest mole values: Cl: 2. 02 = 1 Cl 2. 02 C: H: 2. 02 = 1 C 4. 04 = 2 H 2. 02 4. Write the simplest or empirical formula Timberlake Lecture. PLUS 20 CH 2 Cl

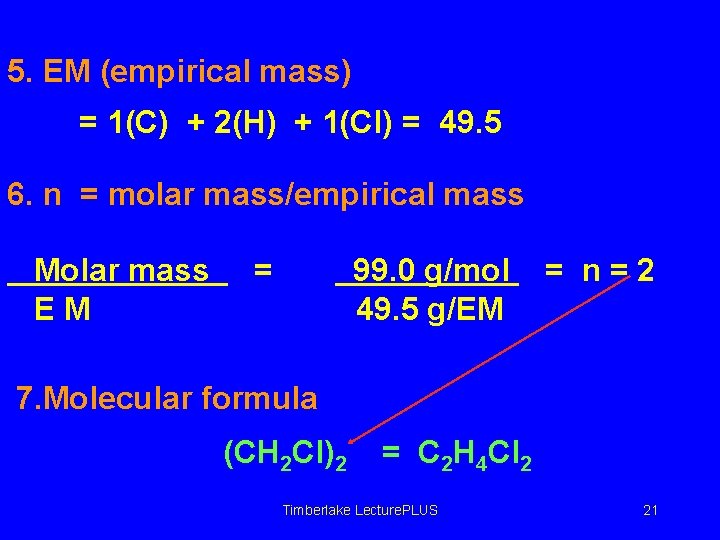
5. EM (empirical mass) = 1(C) + 2(H) + 1(Cl) = 49. 5 6. n = molar mass/empirical mass Molar mass EM = 99. 0 g/mol 49. 5 g/EM = n=2 7. Molecular formula (CH 2 Cl)2 = C 2 H 4 Cl 2 Timberlake Lecture. PLUS 21

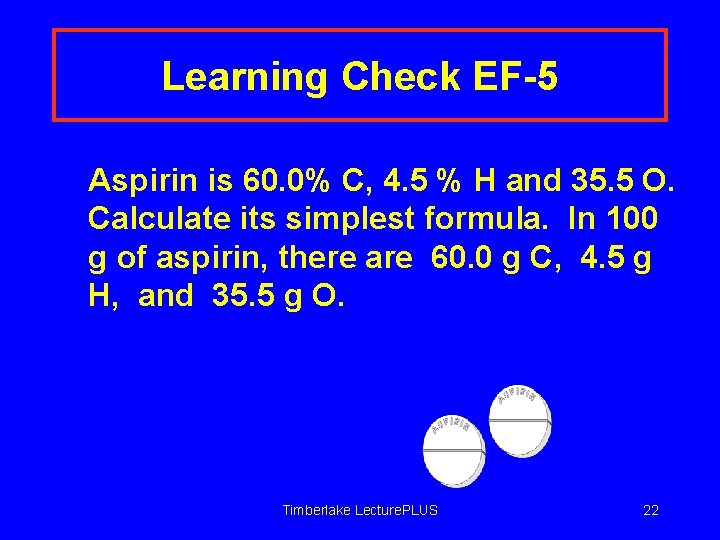
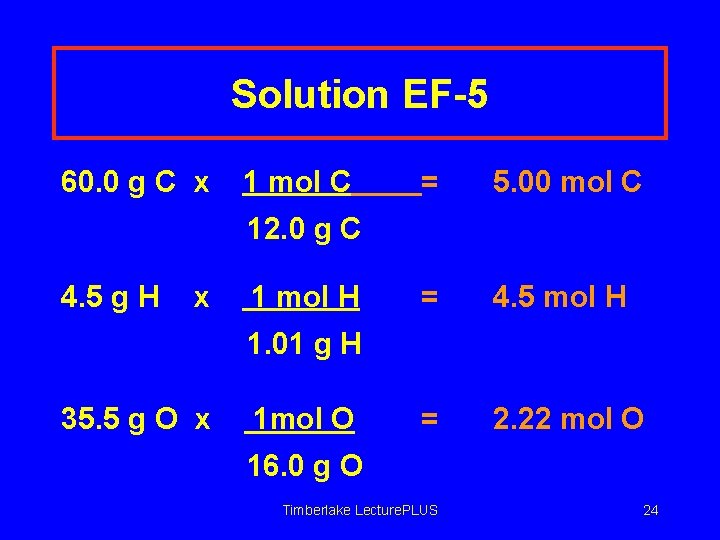
Learning Check EF-5 Aspirin is 60. 0% C, 4. 5 % H and 35. 5 O. Calculate its simplest formula. In 100 g of aspirin, there are 60. 0 g C, 4. 5 g H, and 35. 5 g O. Timberlake Lecture. PLUS 22

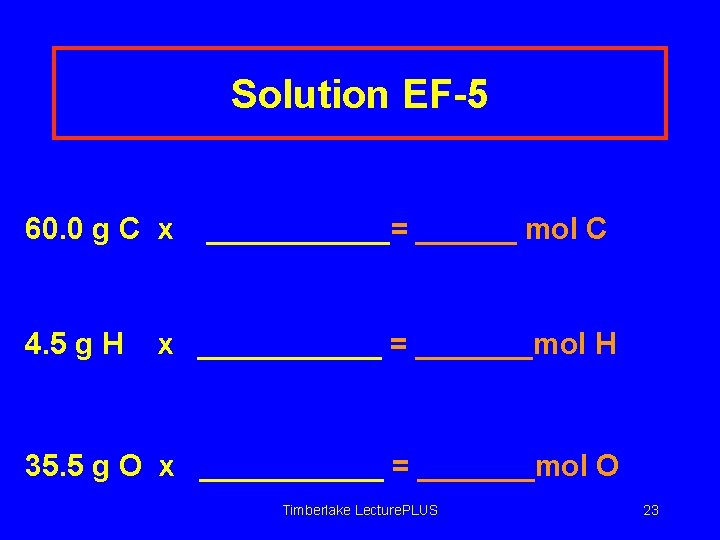
Solution EF-5 60. 0 g C x 4. 5 g H ______= ______ mol C x ______ = _______mol H 35. 5 g O x ______ = _______mol O Timberlake Lecture. PLUS 23

Solution EF-5 60. 0 g C x 1 mol C = 5. 00 mol C = 4. 5 mol H = 2. 22 mol O 12. 0 g C 4. 5 g H x 1 mol H 1. 01 g H 35. 5 g O x 1 mol O 16. 0 g O Timberlake Lecture. PLUS 24

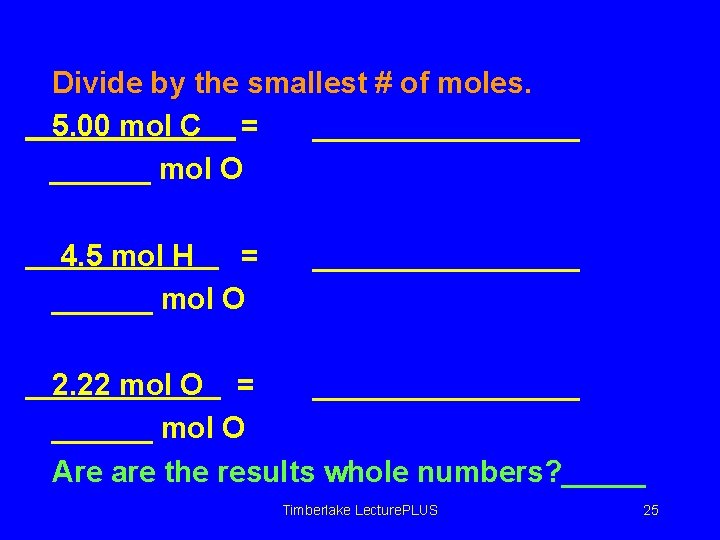
Divide by the smallest # of moles. 5. 00 mol C = ________ mol O 4. 5 mol H = ______ mol O ________ 2. 22 mol O = ________ mol O Are are the results whole numbers? _____ Timberlake Lecture. PLUS 25

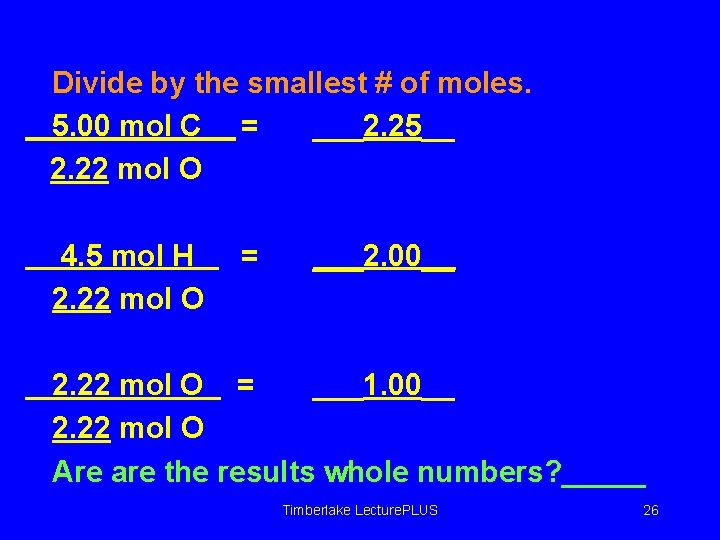
Divide by the smallest # of moles. 5. 00 mol C = ___2. 25__ 2. 22 mol O 4. 5 mol H 2. 22 mol O = ___2. 00__ 2. 22 mol O = ___1. 00__ 2. 22 mol O Are are the results whole numbers? _____ Timberlake Lecture. PLUS 26

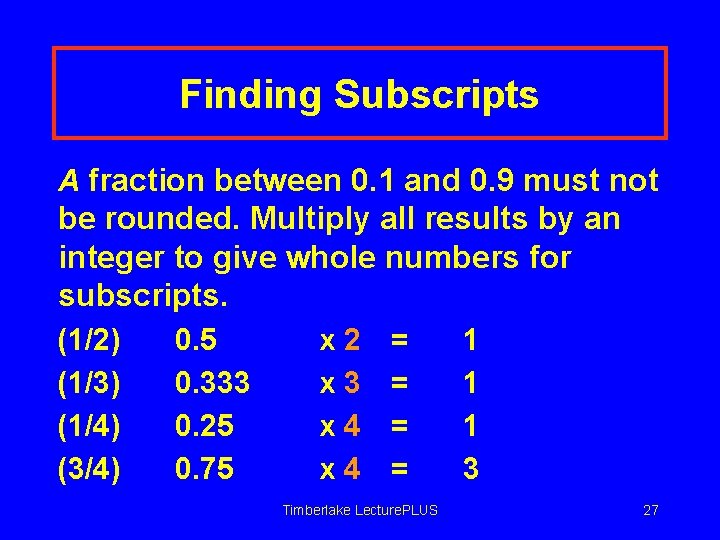
Finding Subscripts A fraction between 0. 1 and 0. 9 must not be rounded. Multiply all results by an integer to give whole numbers for subscripts. (1/2) (1/3) (1/4) (3/4) 0. 5 0. 333 0. 25 0. 75 x 2 x 3 x 4 = = Timberlake Lecture. PLUS 1 1 1 3 27

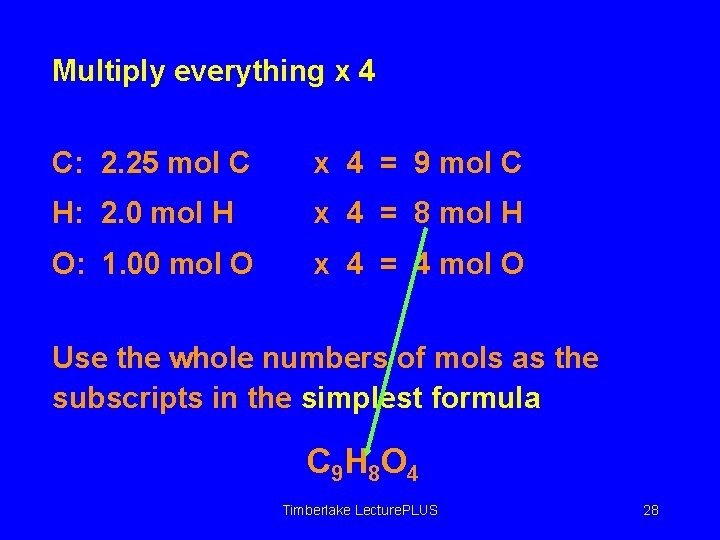
Multiply everything x 4 C: 2. 25 mol C x 4 = 9 mol C H: 2. 0 mol H x 4 = 8 mol H O: 1. 00 mol O x 4 = 4 mol O Use the whole numbers of mols as the subscripts in the simplest formula C 9 H 8 O 4 Timberlake Lecture. PLUS 28

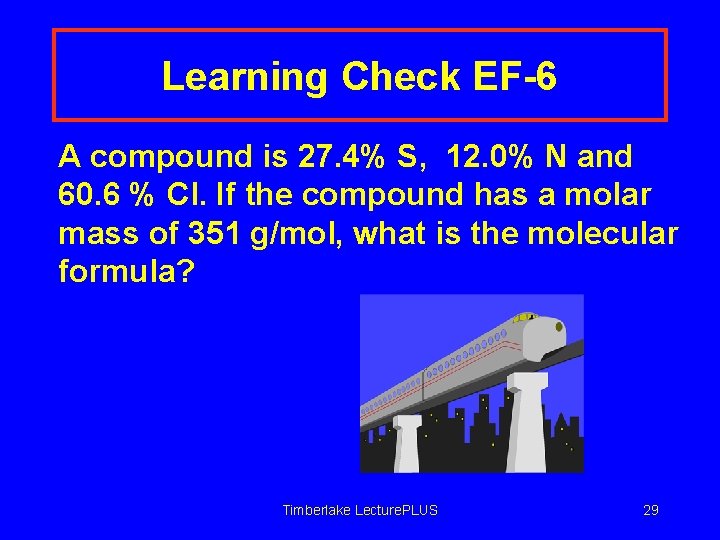
Learning Check EF-6 A compound is 27. 4% S, 12. 0% N and 60. 6 % Cl. If the compound has a molar mass of 351 g/mol, what is the molecular formula? Timberlake Lecture. PLUS 29

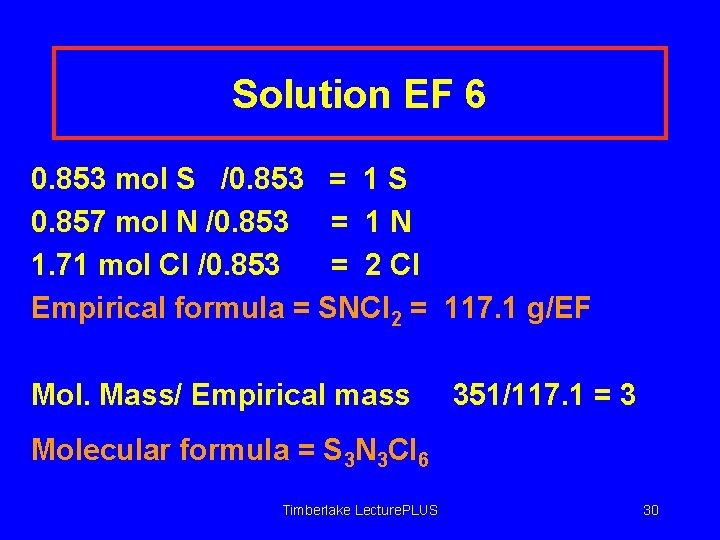
Solution EF 6 0. 853 mol S /0. 853 = 1 S 0. 857 mol N /0. 853 = 1 N 1. 71 mol Cl /0. 853 = 2 Cl Empirical formula = SNCl 2 = 117. 1 g/EF Mol. Mass/ Empirical mass 351/117. 1 = 3 Molecular formula = S 3 N 3 Cl 6 Timberlake Lecture. PLUS 30

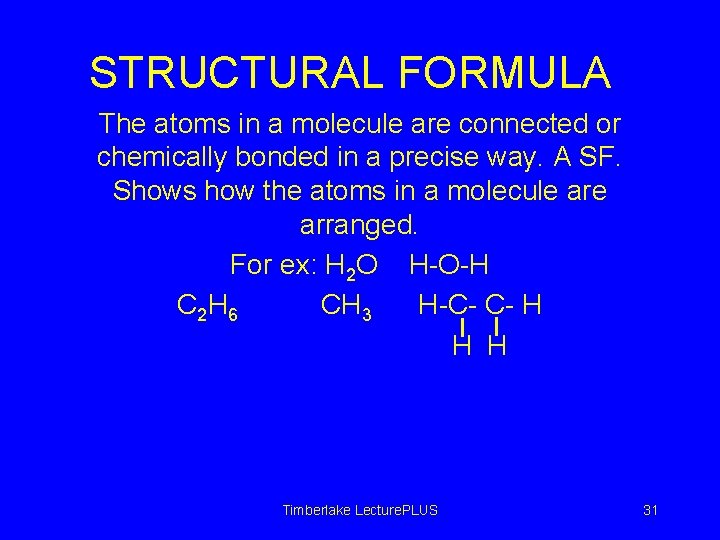
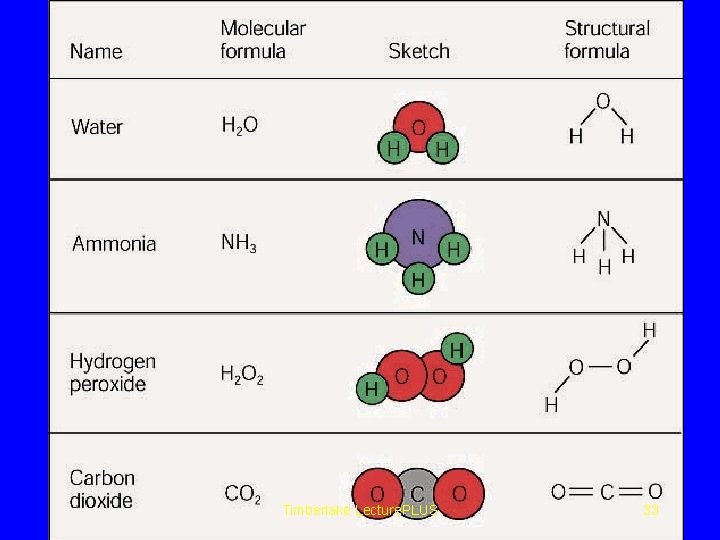
STRUCTURAL FORMULA The atoms in a molecule are connected or chemically bonded in a precise way. A SF. Shows how the atoms in a molecule arranged. For ex: H 2 O H-O-H C 2 H 6 CH 3 H-C- C- H H H Timberlake Lecture. PLUS 31

Empirical formula • The simplest whole number ratio of atoms of elements in a compound, described with the use of subscripts. • Ionic compounds are always shown as empirical formulas. Molecular Formula The actual numbers of atoms in a molecule. Structural Formula Show the relative arrangements of atoms in a molecule Timberlake Lecture. PLUS 32

Timberlake Lecture. PLUS 33

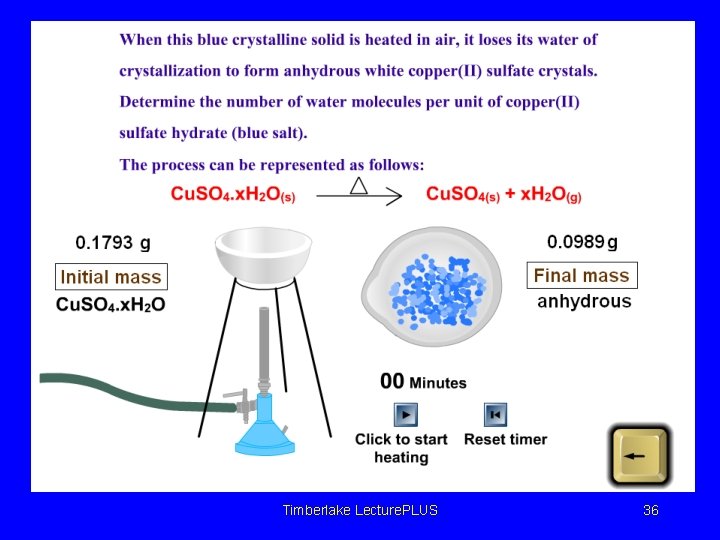
HYDRATES Solids which are found in combined form with water in definite proportion are called as HYDRATES. When hydrates are heated, H 2 O evaporates, and only solid is obtained in amorphous. (w/o a certain geometric structure, generally in powdered form. H 2 O molecules surround ionic substances with certain amounts. Timberlake Lecture. PLUS 34

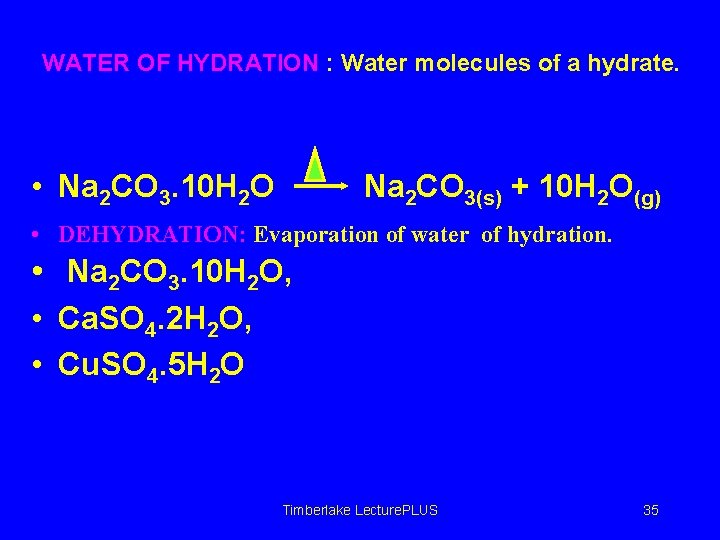
WATER OF HYDRATION : Water molecules of a hydrate. • Na 2 CO 3. 10 H 2 O Na 2 CO 3(s) + 10 H 2 O(g) • DEHYDRATION: Evaporation of water of hydration. • Na 2 CO 3. 10 H 2 O, • Ca. SO 4. 2 H 2 O, • Cu. SO 4. 5 H 2 O Timberlake Lecture. PLUS 35

Timberlake Lecture. PLUS 36