Determine the oxidation states for all atoms in
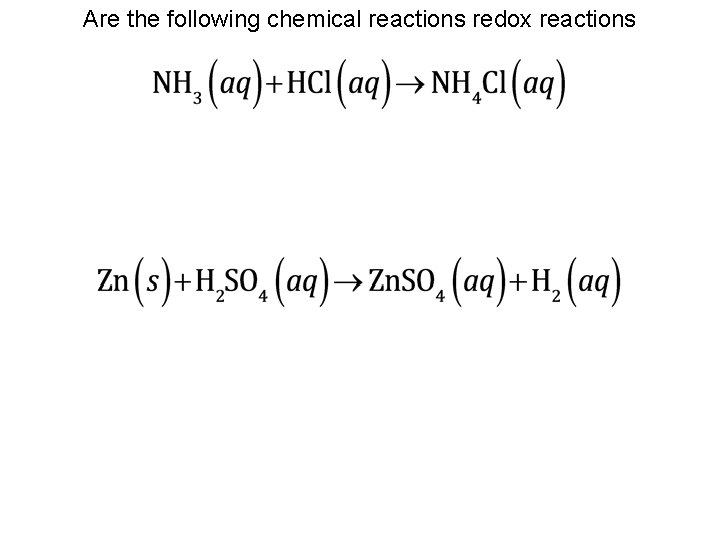
- Slides: 11

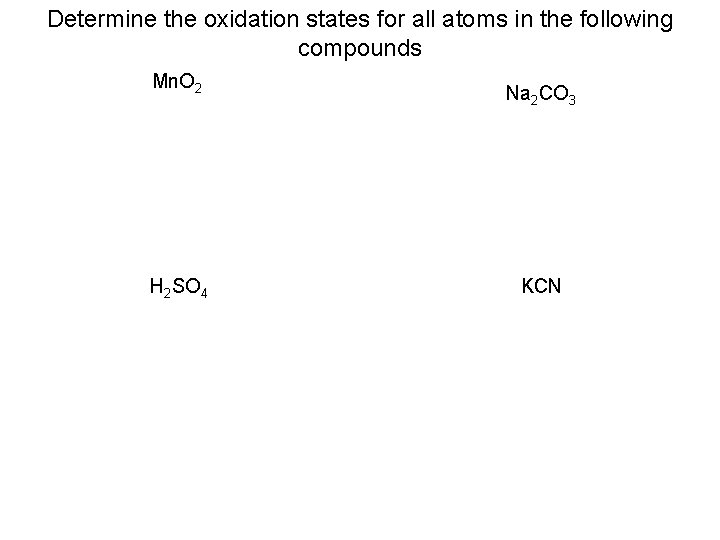
Determine the oxidation states for all atoms in the following compounds Mn. O 2 H 2 SO 4 Na 2 CO 3 KCN

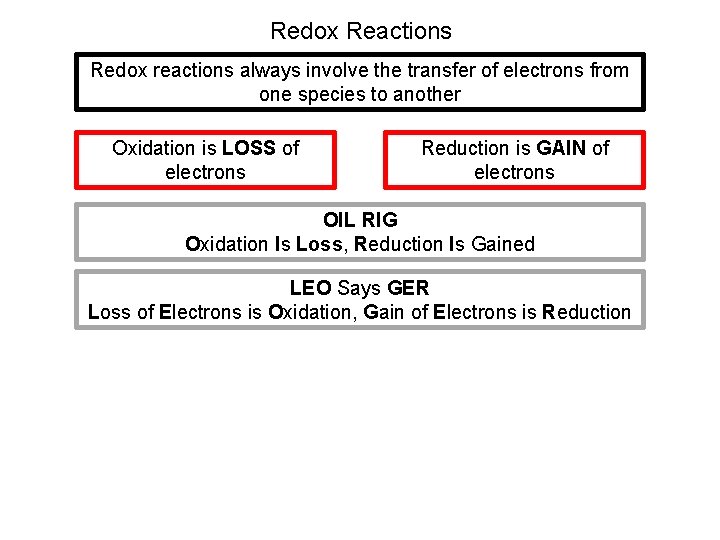
Redox Reactions Redox reactions always involve the transfer of electrons from one species to another Oxidation is LOSS of electrons Reduction is GAIN of electrons OIL RIG Oxidation Is Loss, Reduction Is Gained LEO Says GER Loss of Electrons is Oxidation, Gain of Electrons is Reduction

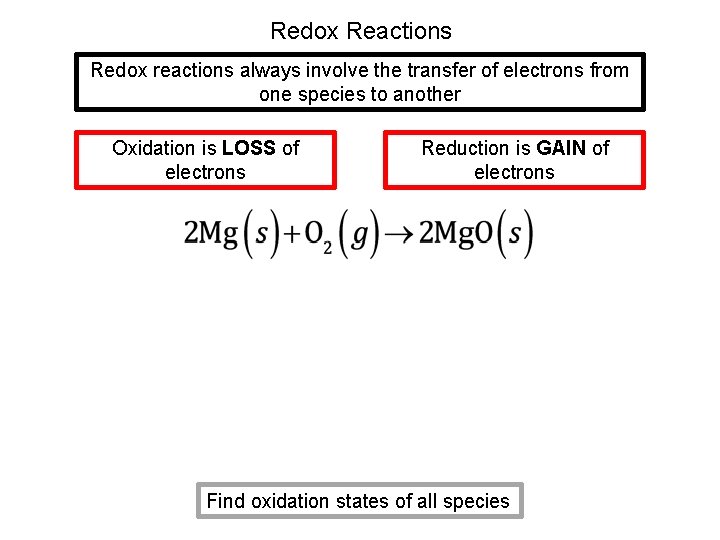
Redox Reactions Redox reactions always involve the transfer of electrons from one species to another Oxidation is LOSS of electrons Reduction is GAIN of electrons Find oxidation states of all species
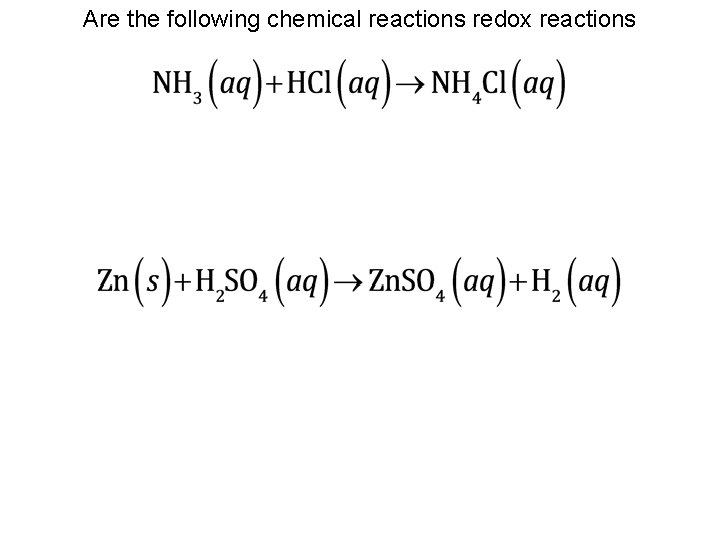
Are the following chemical reactions redox reactions

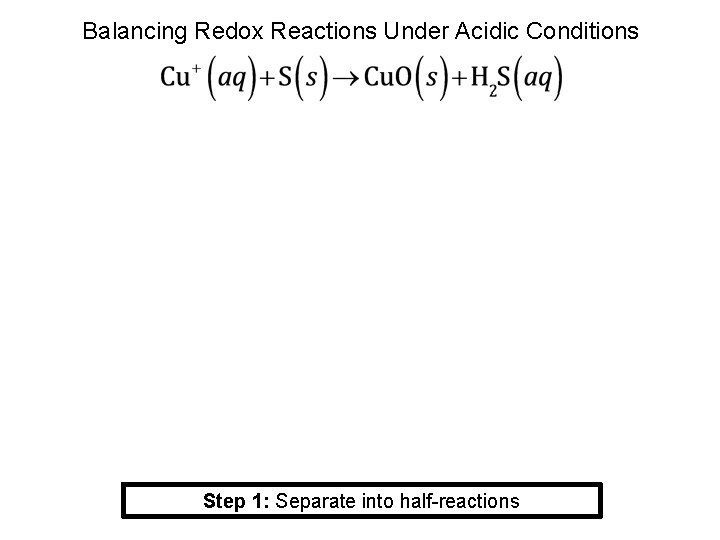
Balancing Redox Reactions Under Acidic Conditions Step 1: Separate into half-reactions

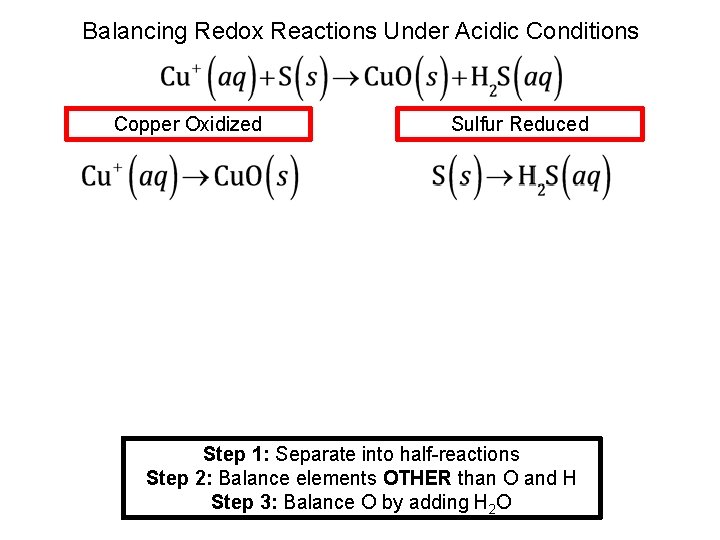
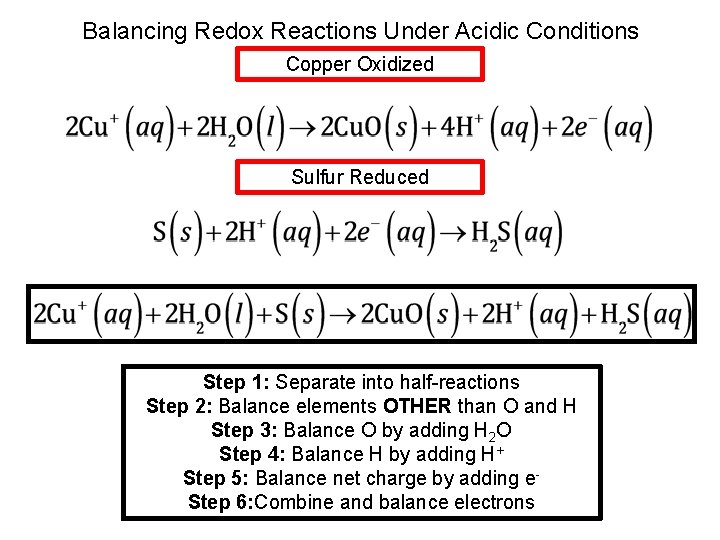
Balancing Redox Reactions Under Acidic Conditions Copper Oxidized Sulfur Reduced Step 1: Separate into half-reactions Step 2: Balance elements OTHER than O and H Step 3: Balance O by adding H 2 O

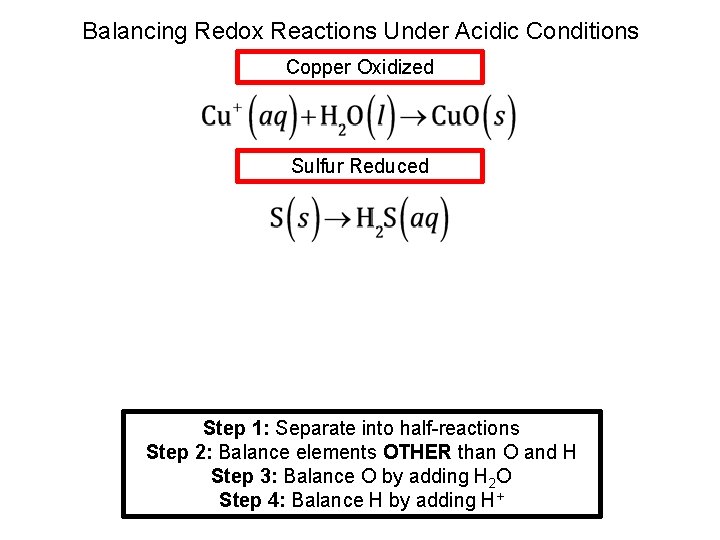
Balancing Redox Reactions Under Acidic Conditions Copper Oxidized Sulfur Reduced Step 1: Separate into half-reactions Step 2: Balance elements OTHER than O and H Step 3: Balance O by adding H 2 O Step 4: Balance H by adding H+

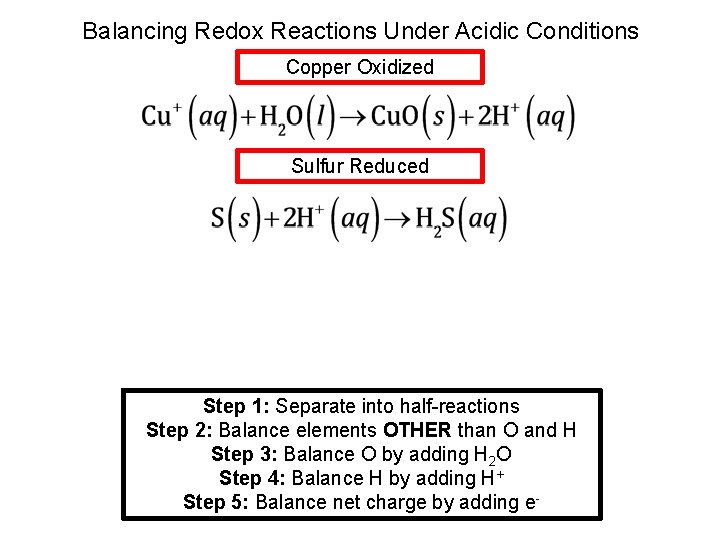
Balancing Redox Reactions Under Acidic Conditions Copper Oxidized Sulfur Reduced Step 1: Separate into half-reactions Step 2: Balance elements OTHER than O and H Step 3: Balance O by adding H 2 O Step 4: Balance H by adding H+ Step 5: Balance net charge by adding e-

Balancing Redox Reactions Under Acidic Conditions Copper Oxidized Sulfur Reduced Step 1: Separate into half-reactions Step 2: Balance elements OTHER than O and H Step 3: Balance O by adding H 2 O Step 4: Balance H by adding H+ Step 5: Balance net charge by adding e. Step 6: Combine and balance electrons

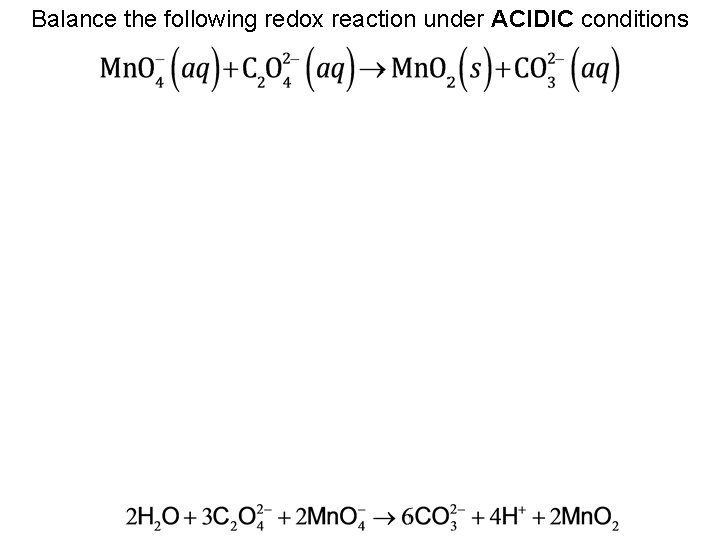
Balance the following redox reaction under ACIDIC conditions

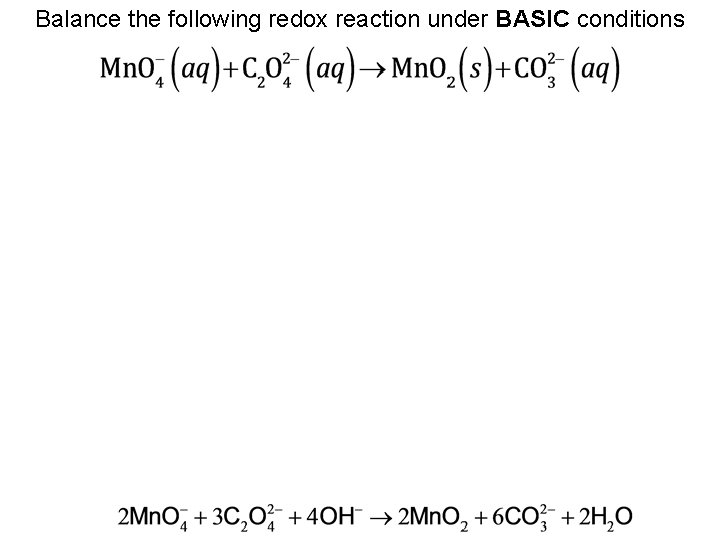
Balance the following redox reaction under BASIC conditions