Determination of the Structure of Cyclopentanone and Argon




- Slides: 27

Determination of the Structure of Cyclopentanone and Argon and Neon Cyclopentanone van der Waals Complexes 40 Ar 36 Ar 20 Ne 22 Ne 18 O 99. 6% 0. 33% 90. 5% 9. 2% 0. 21% Wei Lin*, Andrea J. Minei #, Andrew H. Brooks, Dan Frohman, Chinh H. Duong, Smitty Grubbs, Stewart E. Novick and Wallace C. Pringle Department of Chemistry, Wesleyan University, Middletown, CT *Department of Chemistry University of Texas at Brownsville, TX #Chemistry Department, College of Mount Saint Vincent, Riverdale, NY

Previously Studied van der Waals Complexes • Thietane, oxetane, cyclobutanone, methylene cyclobutene, cyclopentene oxide, chloro-cyclobutane

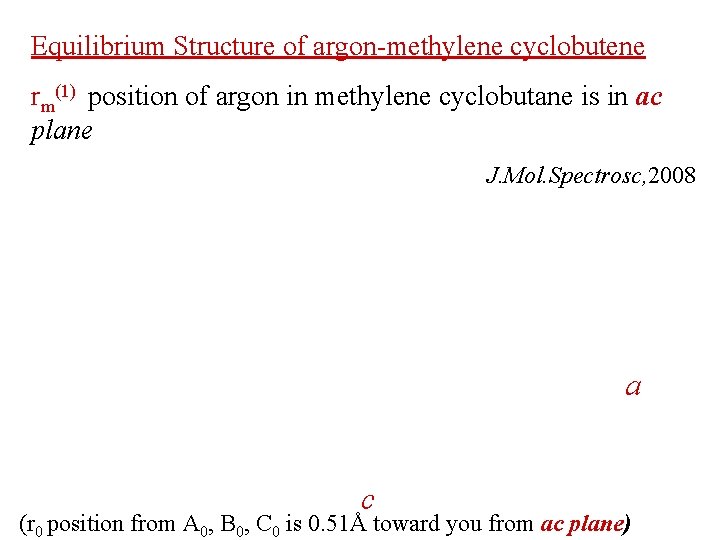
Equilibrium Structure of argon-methylene cyclobutene rm(1) position of argon in methylene cyclobutane is in ac plane J. Mol. Spectrosc, 2008 a c (r 0 position from A 0, B 0, C 0 is 0. 51Å toward you from ac plane)

Rare gas (guest) forms a van der Waals complex with a ring molecule (host) in a collision free supersonic jet at T ~ 2 K a. Where does it attach and why? Lewis base search for pair acceptor (positive part of host) b. The rare gas undergoes 3 very large amplitude motions in its ground state: vd. W stretch, cross ring bend another bend usually in the plane perpendicular to the ring plane. c. The position of the rare gas as determined from an ab initio calculation is at the equilibrium position, re. That is at the minimum in the potential energy for each vibration. d. In rings, this rare gas position is often in the ac plane if the ring. e. The position of the rare gas determined from the observed rotational constants, A 0, B 0, C 0, is not the same as the equilibrium position due to the averaging of the moment of inertia over these very large ground state wavefunctions: <ψ(0)|1/mr 2 |ψ(0)>: this leads to ro structure

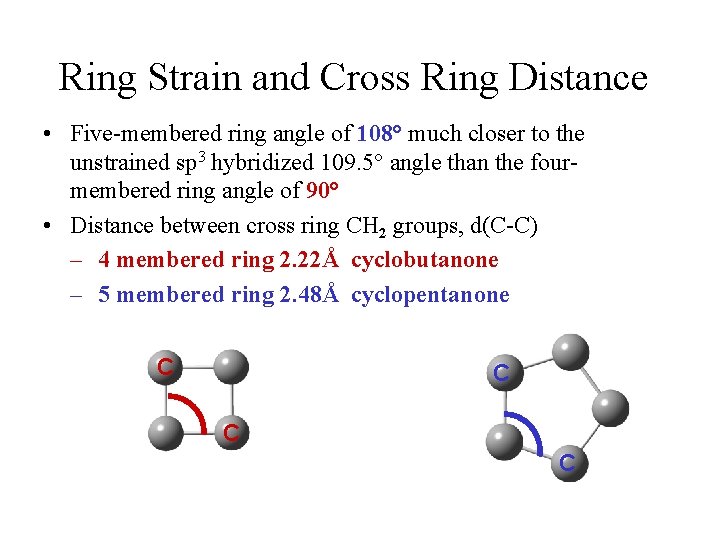
Ring Strain and Cross Ring Distance • Five-membered ring angle of 108° much closer to the unstrained sp 3 hybridized 109. 5° angle than the fourmembered ring angle of 90° • Distance between cross ring CH 2 groups, d(C-C) – 4 membered ring 2. 22Å cyclobutanone – 5 membered ring 2. 48Å cyclopentanone C C

Ring Puckering and Rare Gas Quenching • Ring angle strain is increased if ring is non-planar (108 or 90 is decreased in non-planar ring) • Torsional eclipsed repulsion is reduced if ring becomes non-planar (eclipsed become staggered) • Non-planar rings often have a double minimum inversion vibration: competition tween ring strain {planar} and torsional forces {non-planar} • Some 5 -membered rings exhibit pseudo-rotation • Complexation with rare gas destroys symmetry of double minimum and quenches puckering

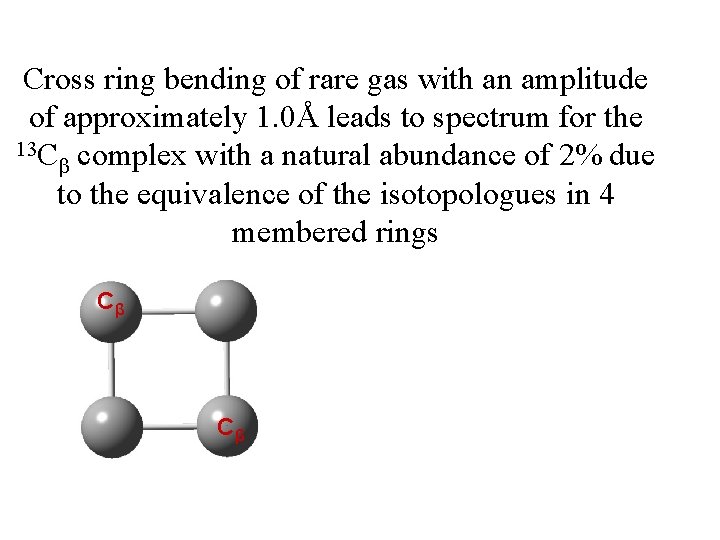
Cross ring bending of rare gas with an amplitude of approximately 1. 0Å leads to spectrum for the 13 C complex with a natural abundance of 2% due β to the equivalence of the isotopologues in 4 membered rings Cβ Cβ

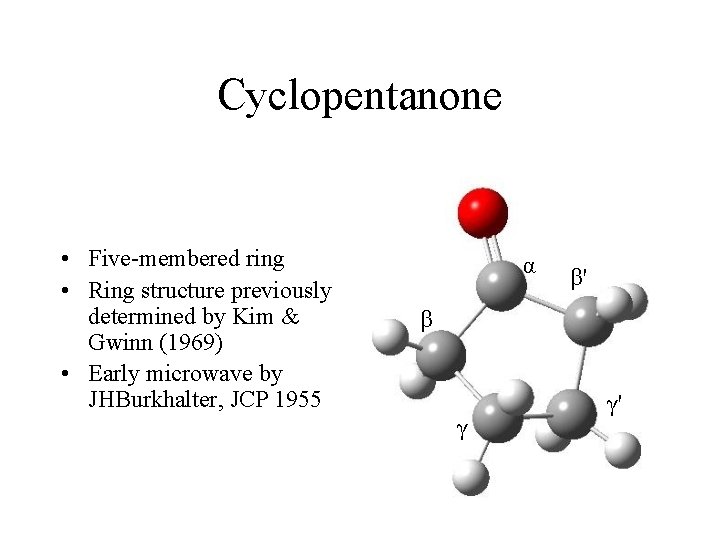
Cyclopentanone • Five-membered ring • Ring structure previously determined by Kim & Gwinn (1969) • Early microwave by JHBurkhalter, JCP 1955 α β' β γ γ'

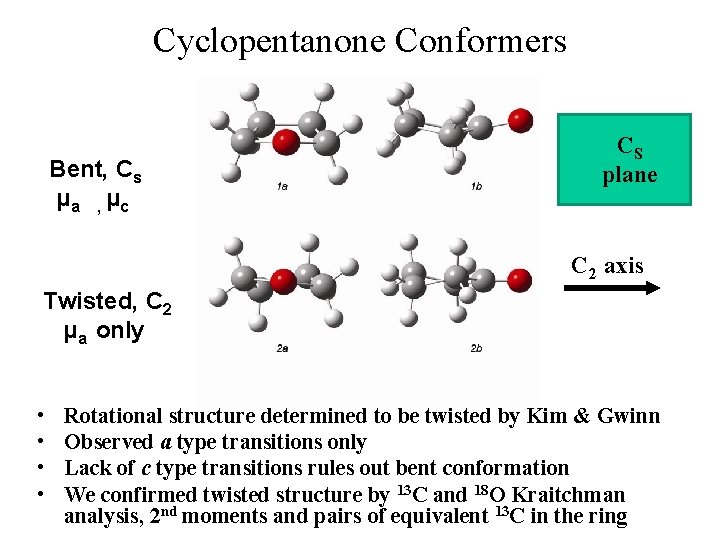
Cyclopentanone Conformers Bent, Cs μa , μc CS plane C 2 axis Twisted, C 2 μa only • • Rotational structure determined to be twisted by Kim & Gwinn Observed a type transitions only Lack of c type transitions rules out bent conformation We confirmed twisted structure by 13 C and 18 O Kraitchman analysis, 2 nd moments and pairs of equivalent 13 C in the ring

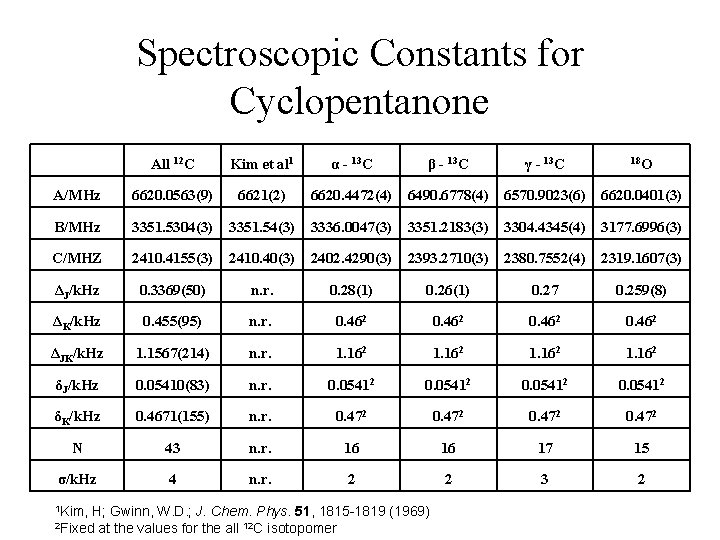
Spectroscopic Constants for Cyclopentanone All 12 C Kim et al 1 α - 13 C β - 13 C γ - 13 C 18 O A/MHz 6620. 0563(9) 6621(2) 6620. 4472(4) 6490. 6778(4) 6570. 9023(6) 6620. 0401(3) B/MHz 3351. 5304(3) 3351. 54(3) 3336. 0047(3) 3351. 2183(3) 3304. 4345(4) 3177. 6996(3) C/MHZ 2410. 4155(3) 2410. 40(3) 2402. 4290(3) 2393. 2710(3) 2380. 7552(4) 2319. 1607(3) ΔJ/k. Hz 0. 3369(50) n. r. 0. 28(1) 0. 26(1) 0. 27 0. 259(8) ΔK/k. Hz 0. 455(95) n. r. 0. 462 ΔJK/k. Hz 1. 1567(214) n. r. 1. 162 δJ/k. Hz 0. 05410(83) n. r. 0. 05412 δK/k. Hz 0. 4671(155) n. r. 0. 472 N 43 n. r. 16 16 17 15 σ/k. Hz 4 n. r. 2 2 3 2 1 Kim, H; Gwinn, W. D. ; J. Chem. Phys. 51, 1815 -1819 (1969) at the values for the all 12 C isotopomer 2 Fixed

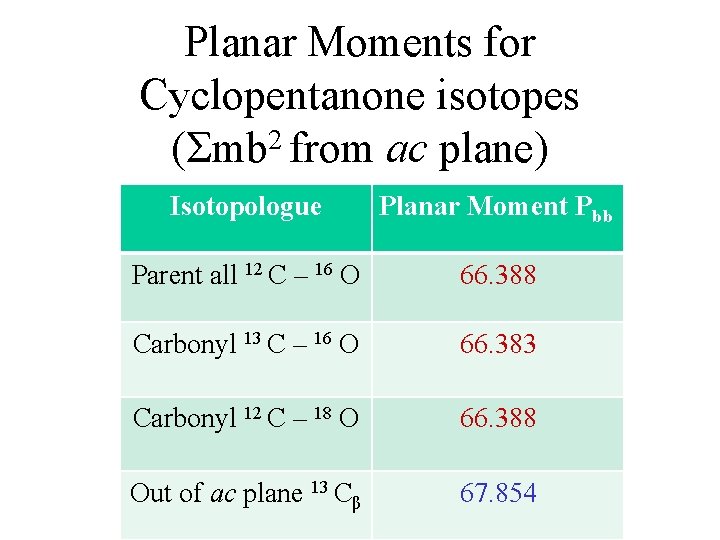
Planar Moments for Cyclopentanone isotopes (Σmb 2 from ac plane) Isotopologue Planar Moment Pbb Parent all 12 C – 16 O 66. 388 Carbonyl 13 C – 16 O 66. 383 Carbonyl 12 C – 18 O 66. 388 Out of ac plane 13 Cβ 67. 854

Cartesian Coordinates of Cyclopentanone

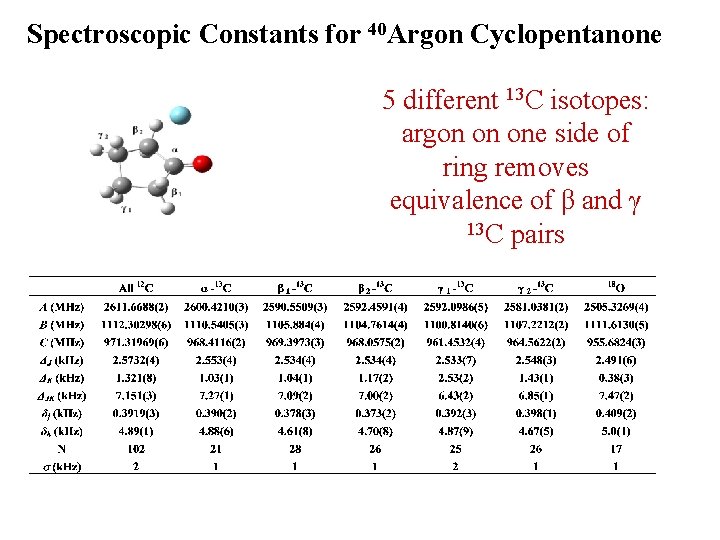
Spectroscopic Constants for 40 Argon Cyclopentanone 5 different 13 C isotopes: argon on one side of ring removes equivalence of β and γ 13 C pairs

Spectroscopic Constants for 36 Argon Cyclopentanone A(MHz) B(MHz) C(MHz) ΔJ (k. Hz) ΔJK (k. Hz) δJ (k. Hz) δK (k. Hz) N 2 a, 7 b σ(k. Hz) 2616. 943 1178. 019 1021. 975 7. 3 -36. 8 0. 392*fixed 40 Ar 4. 88* 9 10

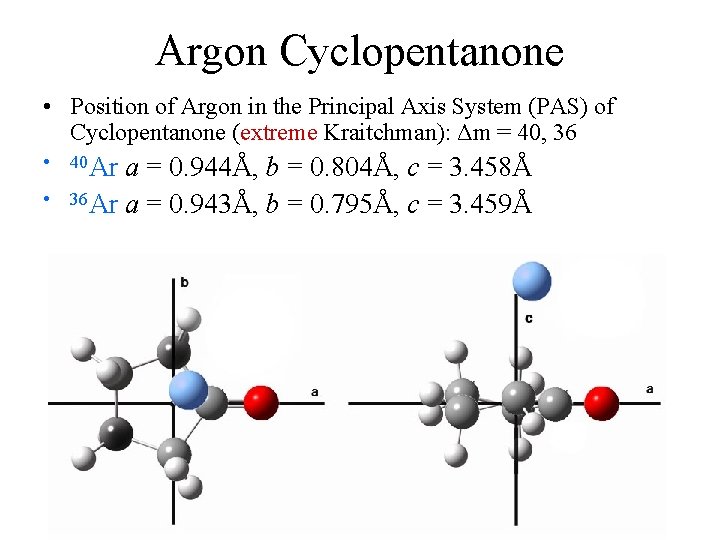
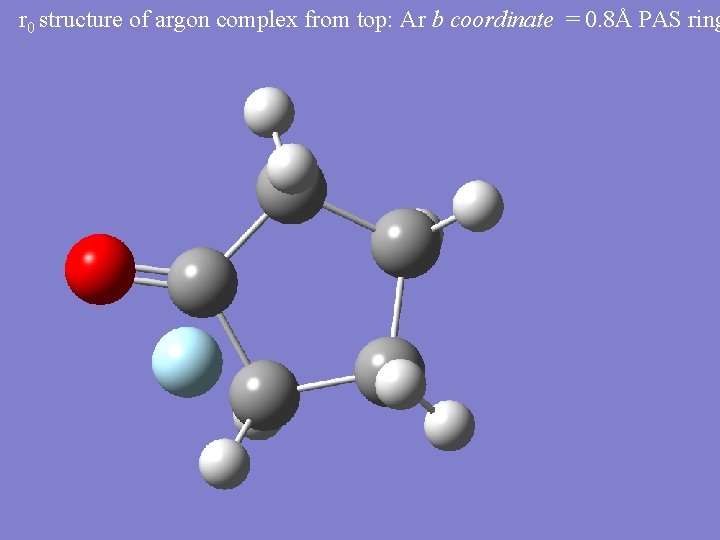
Argon Cyclopentanone • Position of Argon in the Principal Axis System (PAS) of Cyclopentanone (extreme Kraitchman): Δm = 40, 36 • 40 Ar a = 0. 944Å, b = 0. 804Å, c = 3. 458Å • 36 Ar a = 0. 943Å, b = 0. 795Å, c = 3. 459Å

Explain Extreme Kraitchman Assumes the monomer ring is an unsubstituted isotope with rare gas mass = 0. 0 The isotopic substitution is the complex with the rare gas mass equal to 20, 22, 36 or 40 Thus in Kraitchman analysis ΔM = mass of rare gas And the Kraitchman Coordinates are those of the rare gas in the Principal Axis System of the monomer ring Differing vibrational averaging in the monomer and comlex should make coordinates different (especially vd. W bonds) But the coordinates are nearly equal even though mass change is 10%!

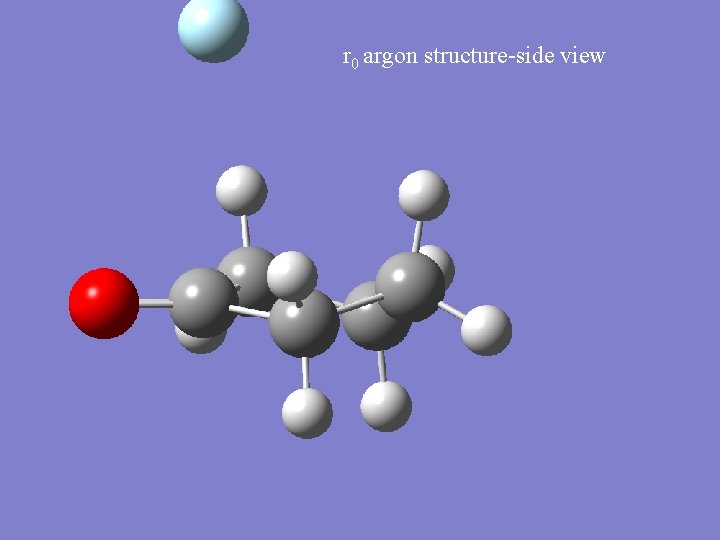
r 0 argon structure-side view

r 0 structure of argon complex from top: Ar b coordinate = 0. 8Å PAS ring

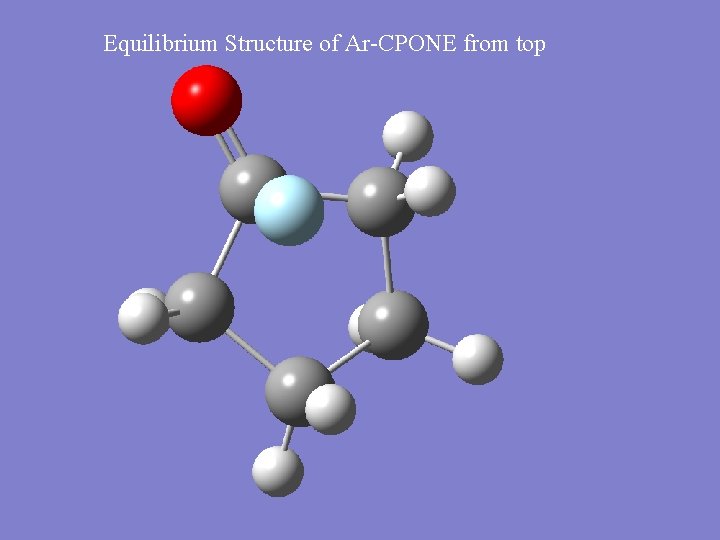
Equilibrium Structure of Ar-CPONE from top

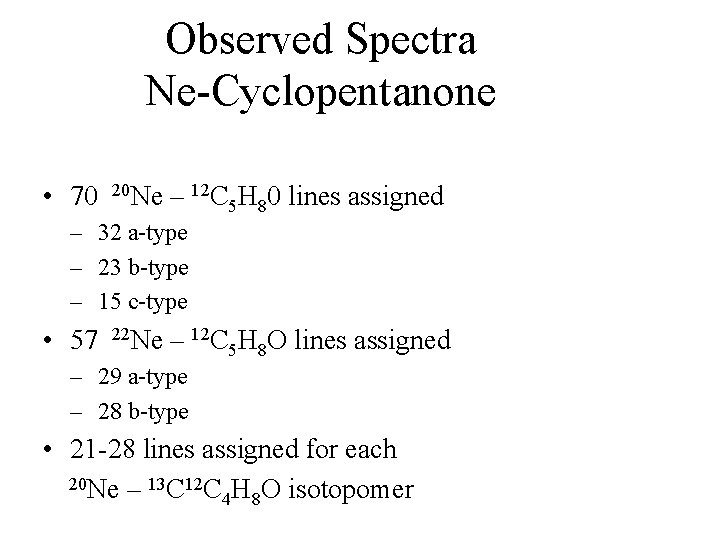
Observed Spectra Ne-Cyclopentanone • 70 20 Ne – 12 C 5 H 80 lines assigned – 32 a-type – 23 b-type – 15 c-type • 57 22 Ne – 12 C 5 H 8 O lines assigned – 29 a-type – 28 b-type • 21 -28 lines assigned for each 20 Ne – 13 C 12 C H O isotopomer 4 8

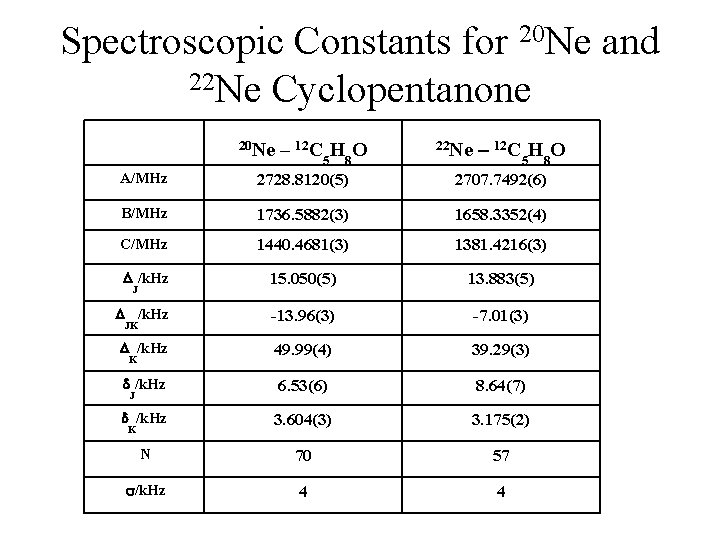
Spectroscopic Constants for 20 Ne and 22 Ne Cyclopentanone 20 Ne – 12 C HO 5 8 22 Ne – 12 C 5 H 8 O A/MHz 2728. 8120(5) 2707. 7492(6) B/MHz 1736. 5882(3) 1658. 3352(4) C/MHz 1440. 4681(3) 1381. 4216(3) /k. Hz 15. 050(5) 13. 883(5) /k. Hz -13. 96(3) -7. 01(3) /k. Hz 49. 99(4) 39. 29(3) /k. Hz 6. 53(6) 8. 64(7) /k. Hz 3. 604(3) 3. 175(2) N 70 57 /k. Hz 4 4 J JK K J K

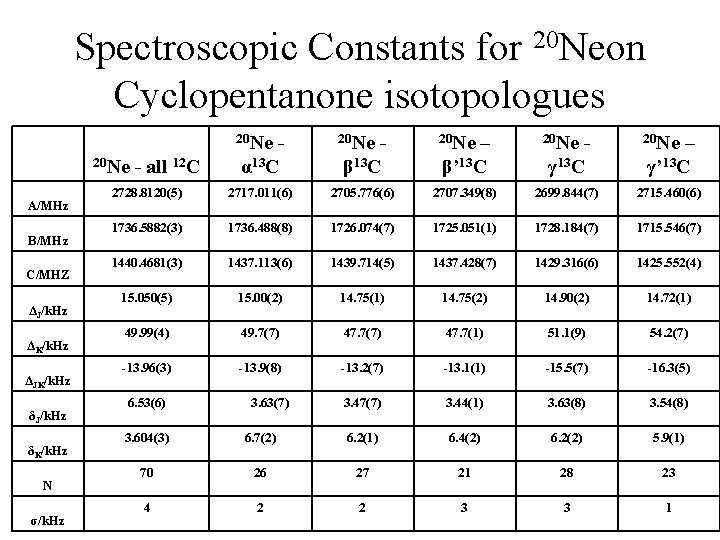
Spectroscopic Constants for 20 Neon Cyclopentanone isotopologues A/MHz B/MHz C/MHZ ΔJ/k. Hz ΔK/k. Hz ΔJK/k. Hz δJ/k. Hz δK/k. Hz N σ/k. Hz 20 Ne - 20 Ne – 20 Ne - all 12 C α 13 C β’ 13 C γ’ 13 C 2728. 8120(5) 2717. 011(6) 2705. 776(6) 2707. 349(8) 2699. 844(7) 2715. 460(6) 1736. 5882(3) 1736. 488(8) 1726. 074(7) 1725. 051(1) 1728. 184(7) 1715. 546(7) 1440. 4681(3) 1437. 113(6) 1439. 714(5) 1437. 428(7) 1429. 316(6) 1425. 552(4) 15. 050(5) 15. 00(2) 14. 75(1) 14. 75(2) 14. 90(2) 14. 72(1) 49. 99(4) 49. 7(7) 47. 7(1) 51. 1(9) 54. 2(7) -13. 96(3) -13. 9(8) -13. 2(7) -13. 1(1) -15. 5(7) -16. 3(5) 6. 53(6) 3. 63(7) 3. 47(7) 3. 44(1) 3. 63(8) 3. 54(8) 3. 604(3) 6. 7(2) 6. 2(1) 6. 4(2) 6. 2(2) 5. 9(1) 70 26 27 21 28 23 4 2 2 3 3 1

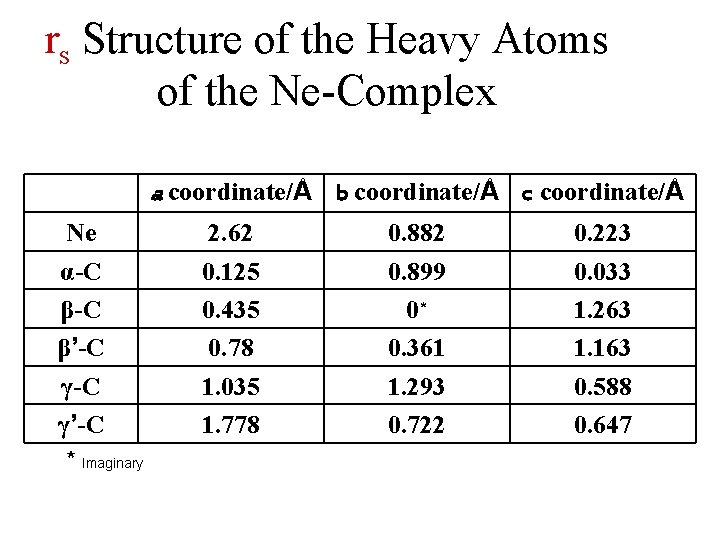
rs Structure of the Heavy Atoms of the Ne-Complex Ne α-C β’-C γ’-C * Imaginary a coordinate/Å b coordinate/Å c coordinate/Å 2. 62 0. 125 0. 435 0. 78 1. 035 1. 778 0. 882 0. 899 0* 0. 361 1. 293 0. 722 0. 223 0. 033 1. 263 1. 163 0. 588 0. 647

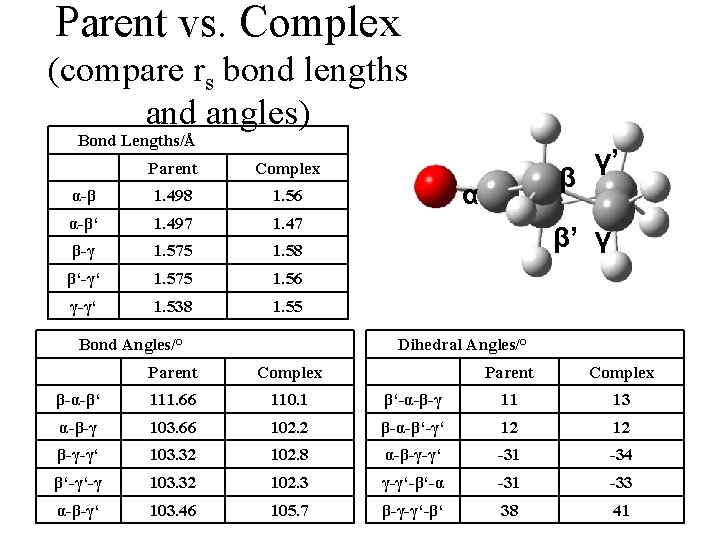
Parent vs. Complex (compare rs bond lengths and angles) Bond Lengths/Å Parent Complex α-β 1. 498 1. 56 α-β‘ 1. 497 1. 47 β-γ 1. 575 1. 58 β‘-γ‘ 1. 575 1. 56 γ-γ‘ 1. 538 1. 55 Bond Angles/° β α γ’ β’ γ Dihedral Angles/° Parent Complex β-α-β‘ 111. 66 110. 1 β‘-α-β-γ 11 13 α-β-γ 103. 66 102. 2 β-α-β‘-γ‘ 12 12 β-γ-γ‘ 103. 32 102. 8 α-β-γ-γ‘ -31 -34 β‘-γ‘-γ 103. 32 102. 3 γ-γ‘-β‘-α -31 -33 α-β-γ‘ 103. 46 105. 7 β-γ-γ‘-β‘ 38 41

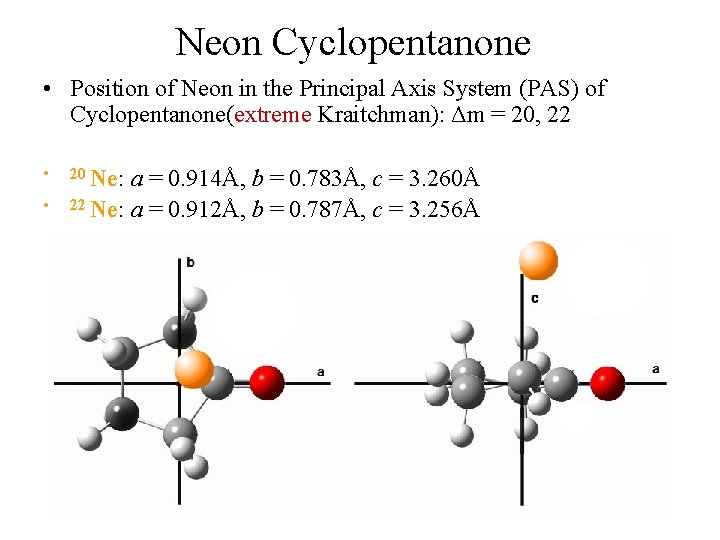
Neon Cyclopentanone • Position of Neon in the Principal Axis System (PAS) of Cyclopentanone(extreme Kraitchman): Δm = 20, 22 • • 20 Ne: a = 0. 914Å, b = 0. 783Å, c = 3. 260Å 22 Ne: a = 0. 912Å, b = 0. 787Å, c = 3. 256Å

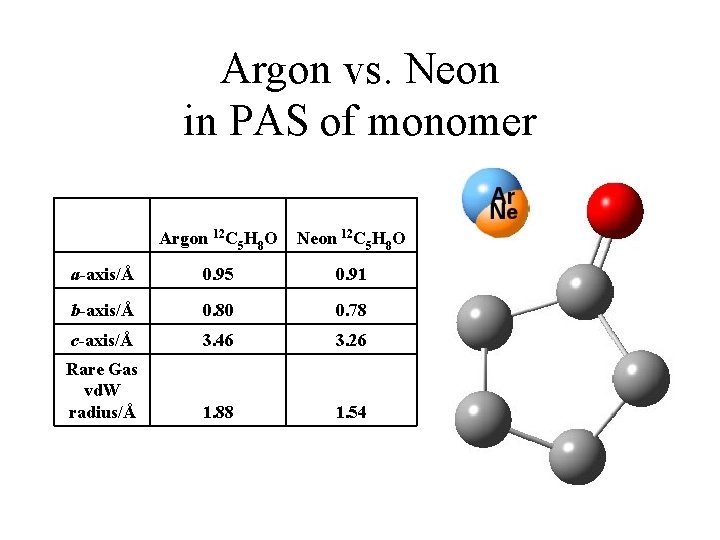
Argon vs. Neon in PAS of monomer Argon 12 C 5 H 8 O Neon 12 C 5 H 8 O a-axis/Å 0. 95 0. 91 b-axis/Å 0. 80 0. 78 c-axis/Å 3. 46 3. 26 Rare Gas vd. W radius/Å 1. 88 1. 54

Conclusions a. Argon and neon complexes with cyclopentanone form over the beta carbon that is below the ab plane of the ring b. Rare gas binding to one side of the ring removes the C 2 symmetry of the ring and 5 unique 13 C complex spectra are observed c. Argon 40 and 36 isotopes have almost exactly the same extreme Kraitchman coordinates in PAS of CPONE d. Neon 20 and 22 isotopes have almost exactly the same extreme Kraitchman coordinates in PAS of CPONE e. The ground state vibrational wave functions for the 3 vd. W large amplitude vibrations do not change much when the mass of the isotopes change by 10%