Detailed Clinical Models for Medical Device Domain Analysis

- Slides: 22

Detailed Clinical Models for Medical Device Domain Analysis Model Catherine Hoang Ioana Singureanu Greg Staudenmaier 1

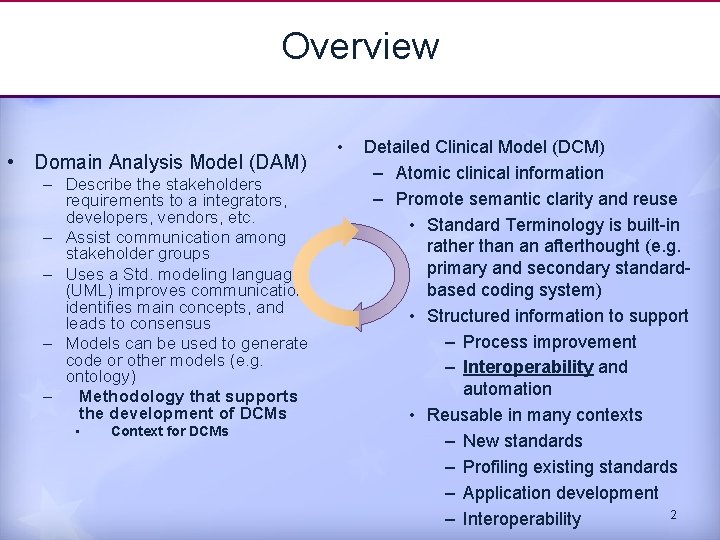
Overview • Domain Analysis Model (DAM) – Describe the stakeholders requirements to a integrators, developers, vendors, etc. – Assist communication among stakeholder groups – Uses a Std. modeling language (UML) improves communication, identifies main concepts, and leads to consensus – Models can be used to generate code or other models (e. g. ontology) – Methodology that supports the development of DCMs • Context for DCMs • Detailed Clinical Model (DCM) – Atomic clinical information – Promote semantic clarity and reuse • Standard Terminology is built-in rather than an afterthought (e. g. primary and secondary standardbased coding system) • Structured information to support – Process improvement – Interoperability and automation • Reusable in many contexts – New standards – Profiling existing standards – Application development 2 – Interoperability

Glossary • DAM: Domain Analysis Model • UML: Unified Modeling Language – a standard developed by the Object Management Group • DCM: Detailed Clinical Model – reusable information models, standardized • LOINC: Logical Observation Identifiers Names and Codes – standard codes for laboratory • SNOMED-CT: Systematized Nomenclature of Medicine-- • ISO: International Organization for Standardization – standards development organization • HL 7: Health Level Sevenhealthcare interoperability standards development organization • IHE: Integrating the Healthcare Enterprise – standards-related consortium • Continua Health Alliance – standards-related consortium specialized in personal 3

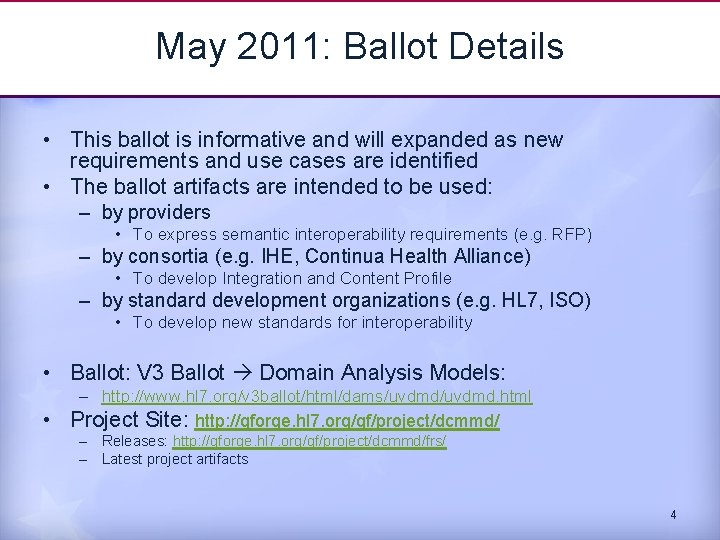
May 2011: Ballot Details • This ballot is informative and will expanded as new requirements and use cases are identified • The ballot artifacts are intended to be used: – by providers • To express semantic interoperability requirements (e. g. RFP) – by consortia (e. g. IHE, Continua Health Alliance) • To develop Integration and Content Profile – by standard development organizations (e. g. HL 7, ISO) • To develop new standards for interoperability • Ballot: V 3 Ballot Domain Analysis Models: • – http: //www. hl 7. org/v 3 ballot/html/dams/uvdmd. html Project Site: http: //gforge. hl 7. org/gf/project/dcmmd/ – Releases: http: //gforge. hl 7. org/gf/project/dcmmd/frs/ – Latest project artifacts 4

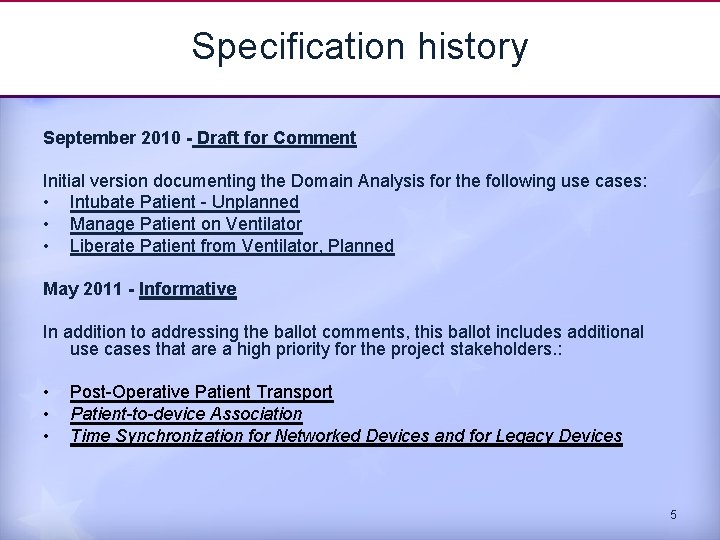
Specification history September 2010 - Draft for Comment Initial version documenting the Domain Analysis for the following use cases: • Intubate Patient - Unplanned • Manage Patient on Ventilator • Liberate Patient from Ventilator, Planned May 2011 - Informative In addition to addressing the ballot comments, this ballot includes additional use cases that are a high priority for the project stakeholders. : • • • Post-Operative Patient Transport Patient-to-device Association Time Synchronization for Networked Devices and for Legacy Devices 5

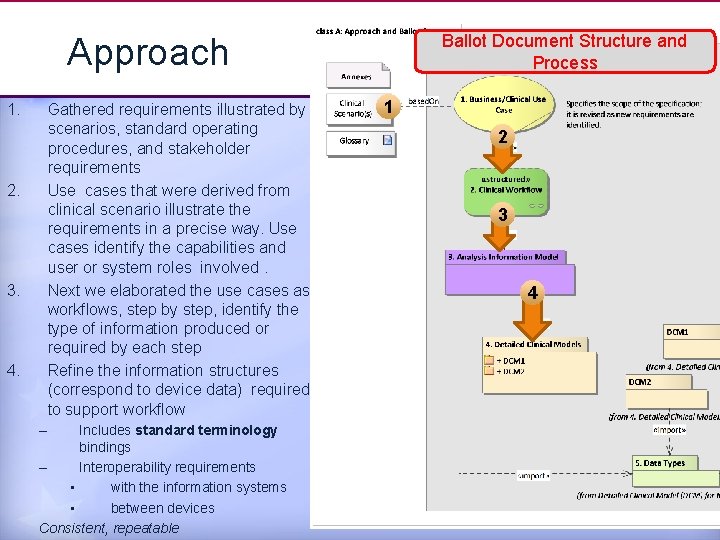
Ballot Document Structure and Process Approach 1. Gathered requirements illustrated by scenarios, standard operating procedures, and stakeholder requirements Use cases that were derived from clinical scenario illustrate the requirements in a precise way. Use cases identify the capabilities and user or system roles involved. Next we elaborated the use cases as workflows, step by step, identify the type of information produced or required by each step Refine the information structures (correspond to device data) required to support workflow 2. 3. 4. 1 2 3 4 – Includes standard terminology bindings – Interoperability requirements • with the information systems • between devices Consistent, repeatable 6

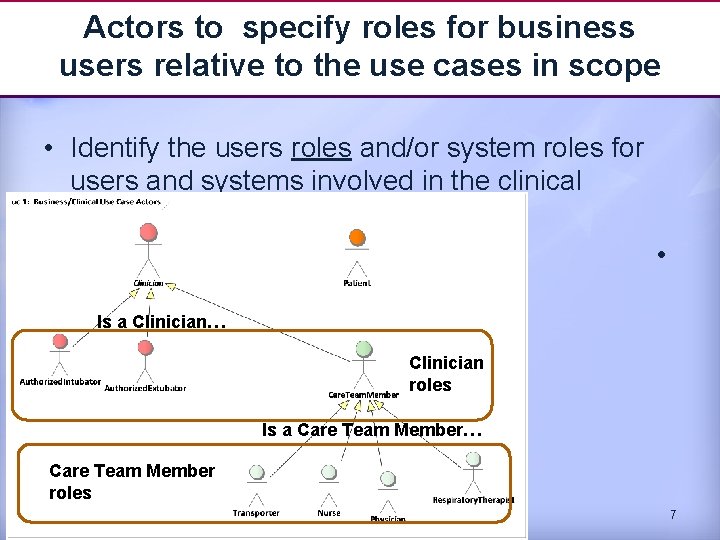
Actors to specify roles for business users relative to the use cases in scope • Identify the users roles and/or system roles for users and systems involved in the clinical scenario(s) • Is a Clinician… Clinician roles Is a Care Team Member… Care Team Member roles 7

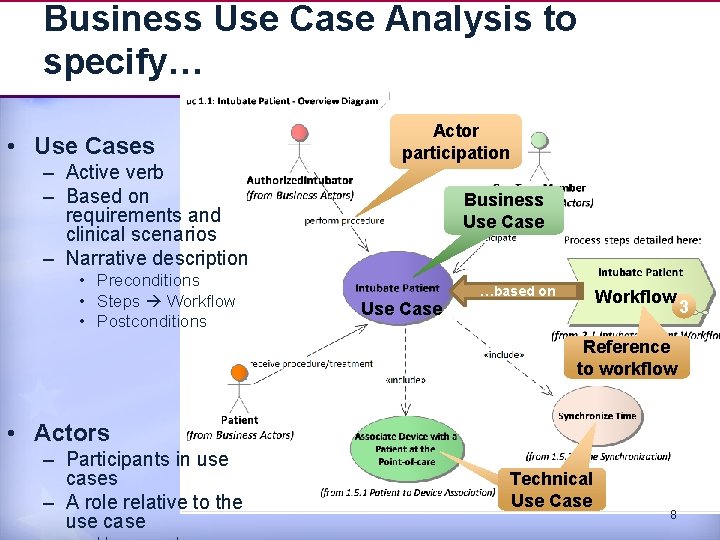
Business Use Case Analysis to specify… • Use Cases – Active verb – Based on requirements and clinical scenarios – Narrative description • Preconditions • Steps Workflow • Postconditions Actor participation Business Use Case …based on Workflow Reference to workflow • Actors – Participants in use cases – A role relative to the use case Technical Use Case 8 3

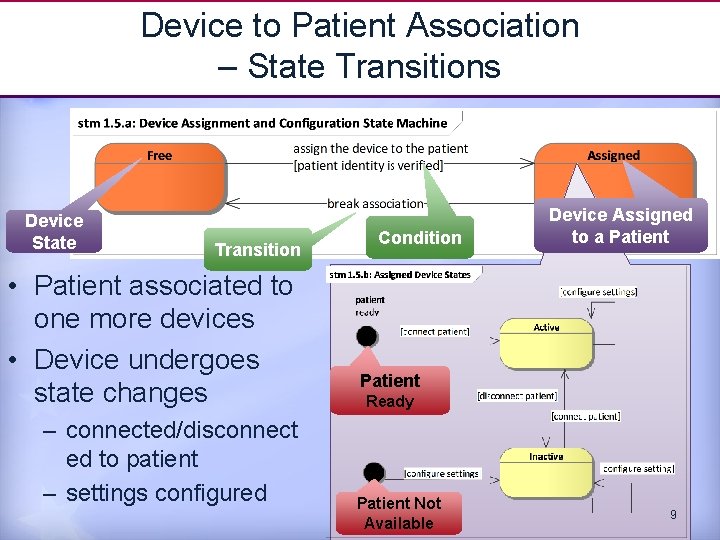
Device to Patient Association – State Transitions Device State Transition • Patient associated to one more devices • Device undergoes state changes – connected/disconnect ed to patient – settings configured Condition Device Assigned to a Patient Ready Patient Not Available 9

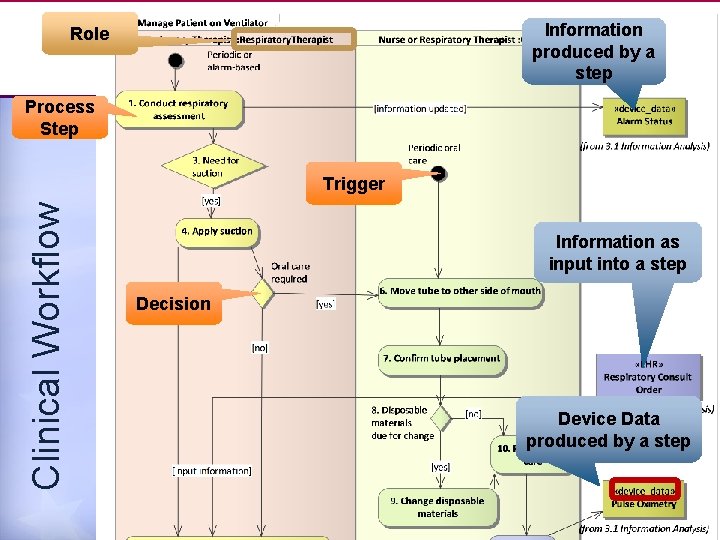
Information produced by a step Role Process Step Clinical Workflow Trigger Information as input into a step Decision Device Data produced by a step 10

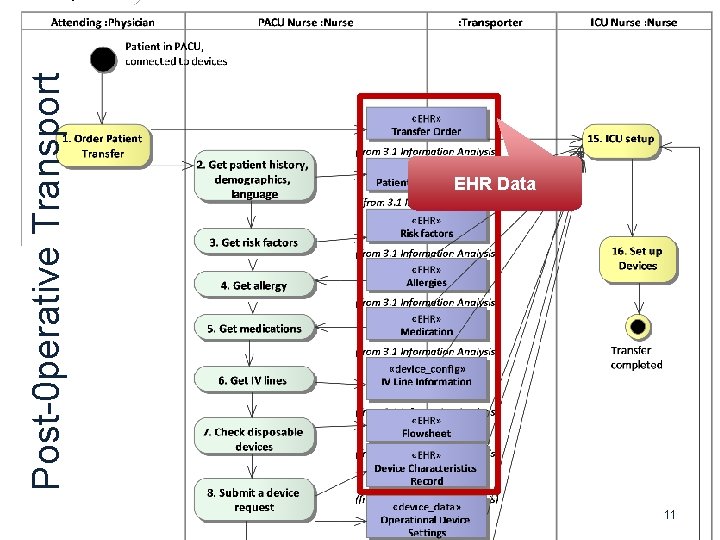
Post-0 perative Transport EHR Data 11

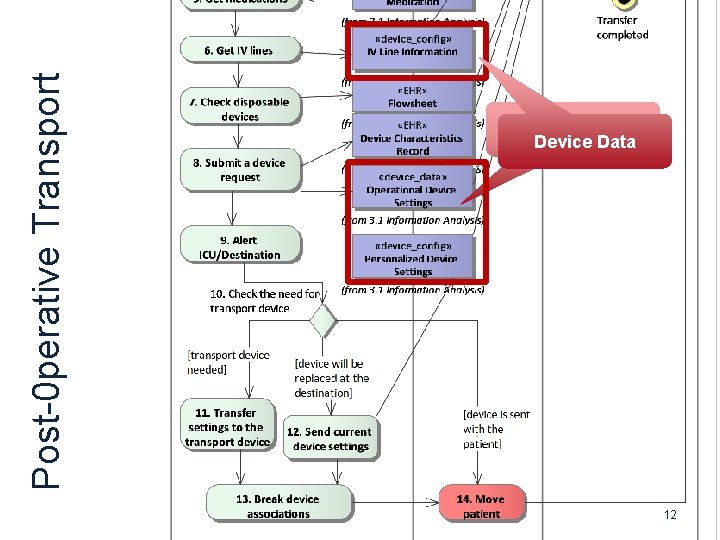
Post-0 perative Transport Device Data 12

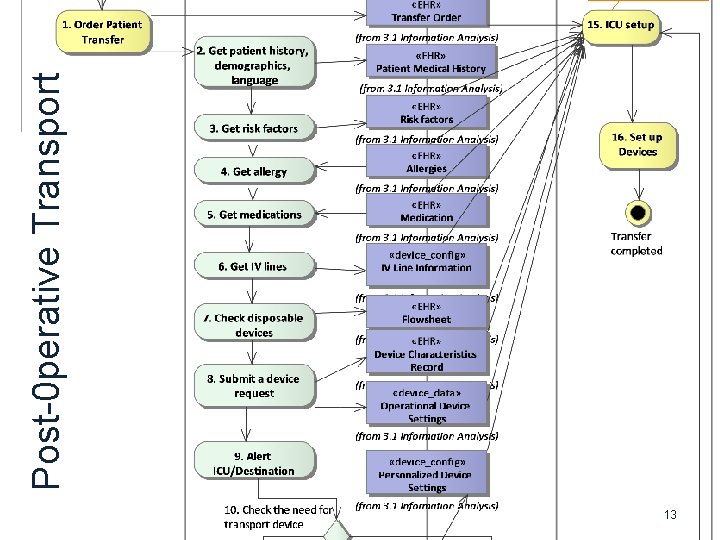
13 Post-0 perative Transport

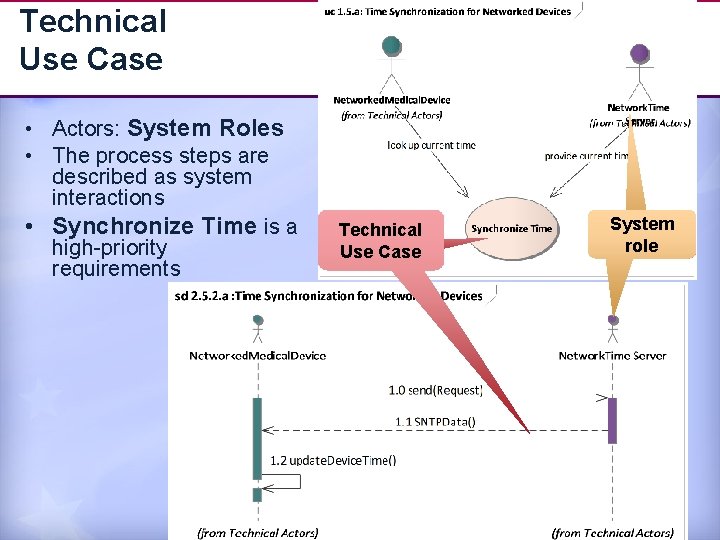
Technical Use Case • Actors: System Roles • The process steps are described as system interactions • Synchronize Time is a high-priority requirements Technical al Use Case System role 14

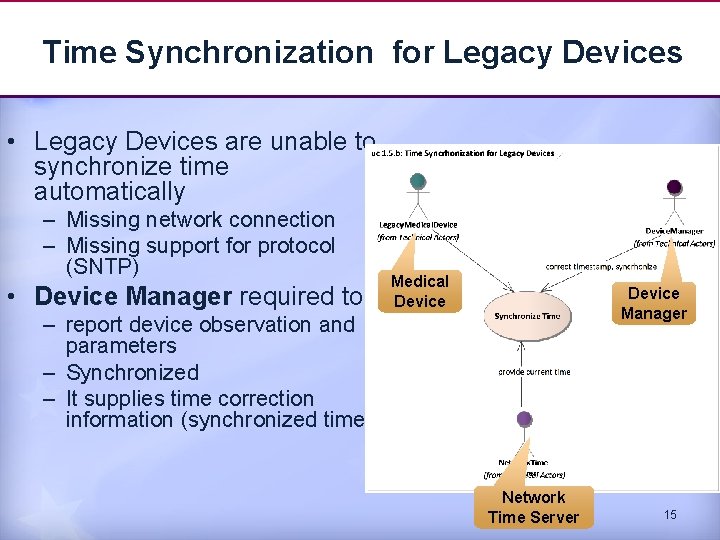
Time Synchronization for Legacy Devices • Legacy Devices are unable to synchronize time automatically – Missing network connection – Missing support for protocol (SNTP) • Device Manager required to Medical Device Manager – report device observation and parameters – Synchronized – It supplies time correction information (synchronized time) Network Time Server 15

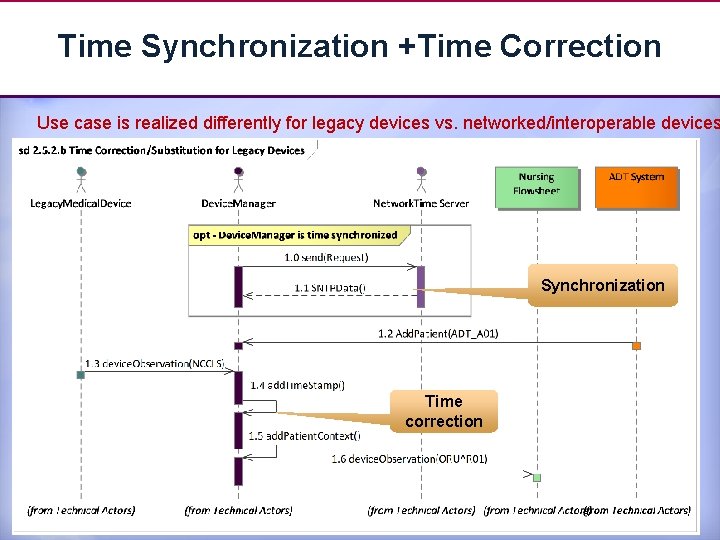
Time Synchronization +Time Correction Use case is realized differently for legacy devices vs. networked/interoperable devices Synchronization Time correction 16

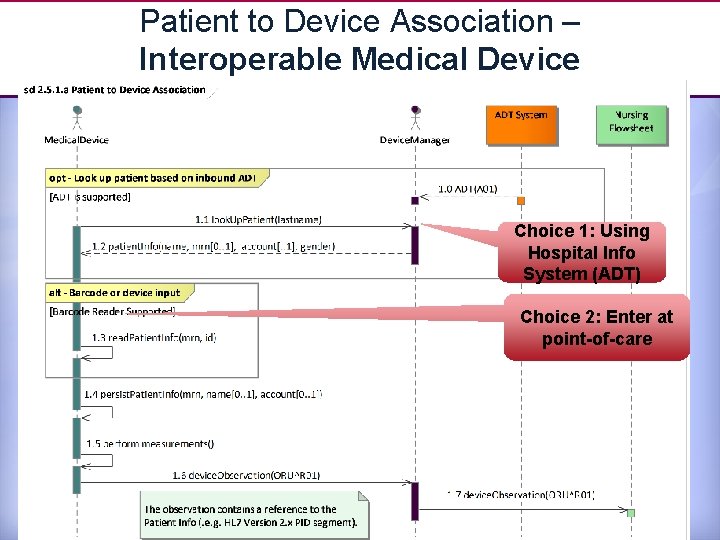
Patient to Device Association – Interoperable Medical Device Choice 1: Using Hospital Info System (ADT) Choice 2: Enter at point-of-care 17

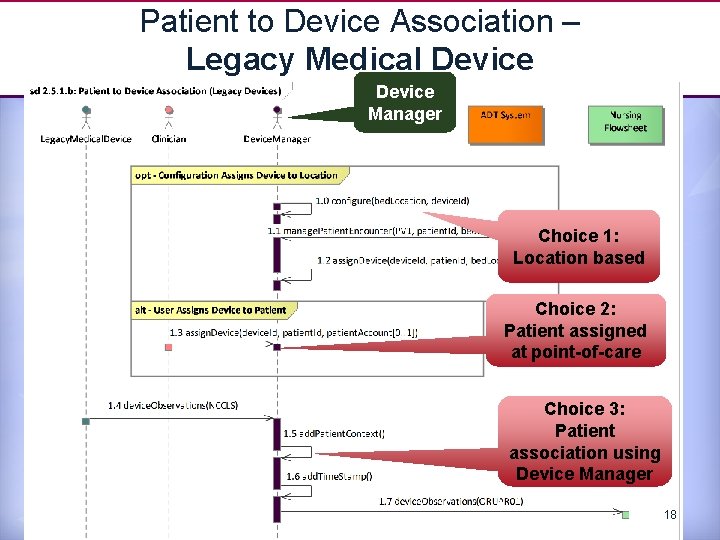
Patient to Device Association – Legacy Medical Device Manager Choice 1: Location based Choice 2: Patient assigned at point-of-care Choice 3: Patient association using Device Manager 18

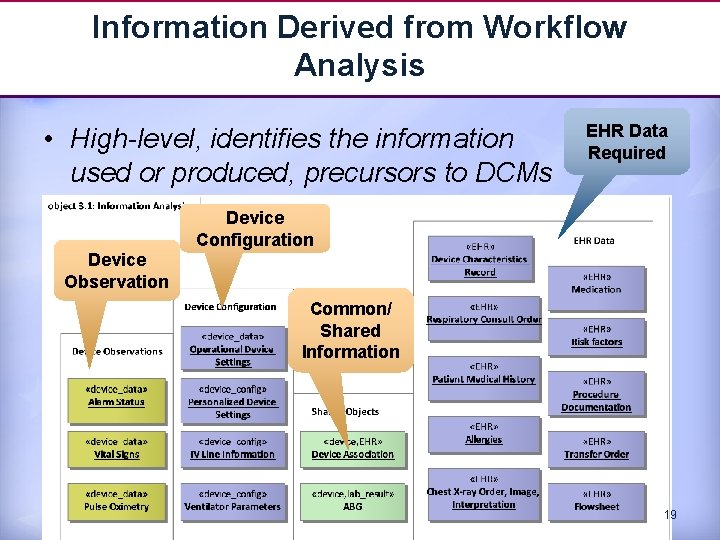
Information Derived from Workflow Analysis • High-level, identifies the information used or produced, precursors to DCMs EHR Data Required Device Configuration Device Observation Common/ Shared Information 19

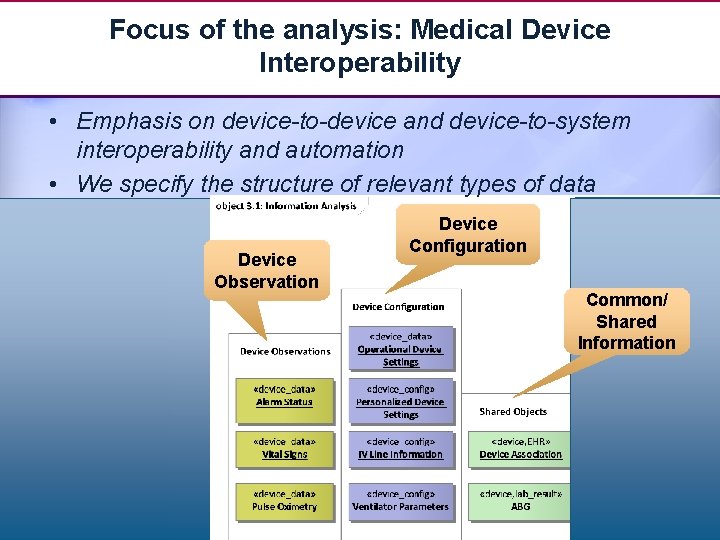
Focus of the analysis: Medical Device Interoperability • Emphasis on device-to-device and device-to-system interoperability and automation • We specify the structure of relevant types of data Device Observation Device Configuration Common/ Shared Information 20

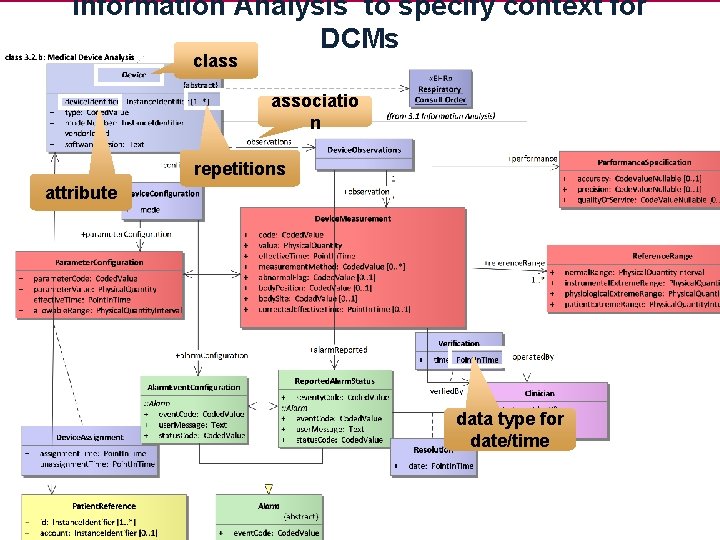
Information Analysis to specify context for DCMs class associatio n repetitions attribute data type for date/time 21

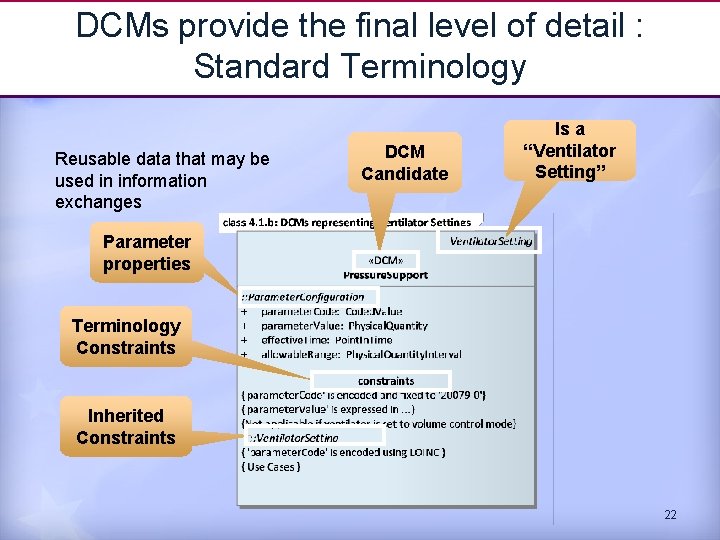
DCMs provide the final level of detail : Standard Terminology Reusable data that may be used in information exchanges DCM Candidate Is a “Ventilator Setting” Parameter properties Terminology Constraints Inherited Constraints 22