Design of Novel Prodrugs for the Treatment of







- Slides: 19

Design of Novel Pro-drugs for the Treatment of Cystinosis Dr D. Cairns Institute of Pharmacy and Chemistry University of Sunderland, UK

Nephropathic cystinosis Rare autosomal recessive metabolic disease n Characterized by elevated levels of intracellular cystine n Caused by defect in lysosomal transporter mechanism for cystine n CTNS gene described n

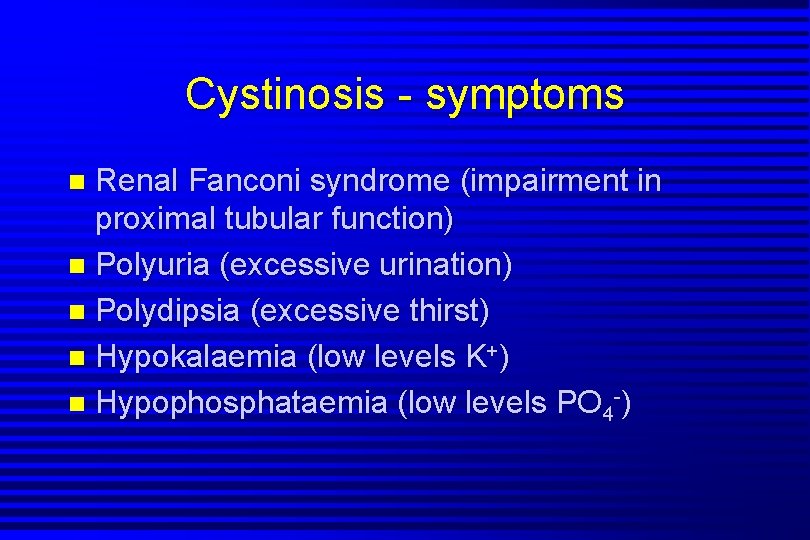
Cystinosis - symptoms Renal Fanconi syndrome (impairment in proximal tubular function) n Polyuria (excessive urination) n Polydipsia (excessive thirst) n Hypokalaemia (low levels K+) n Hypophosphataemia (low levels PO 4 -) n

Cystinosis - symptoms Crystals of cystine present in lysosomes, bone marrow aspirates, leukocytes, cornea and conjunctiva n Photophobia, headaches, burning/itching of eyes n Growth retardation, rickets, muscle myopathy n CNS involvement, hypothyroidism n Hepatic and GI complications n

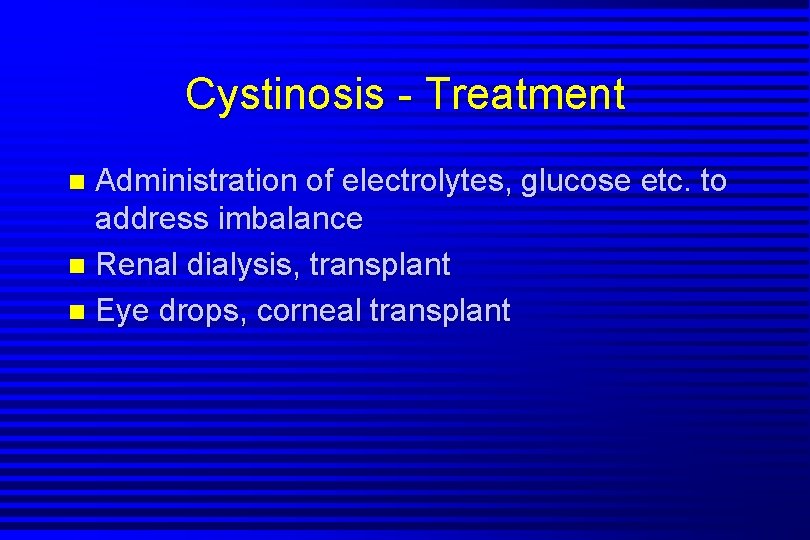
Cystinosis - Treatment Administration of electrolytes, glucose etc. to address imbalance n Renal dialysis, transplant n Eye drops, corneal transplant n

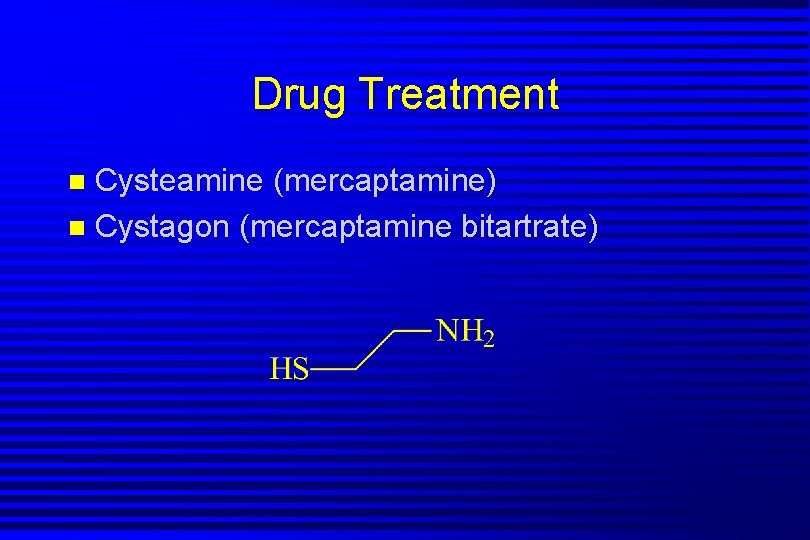
Drug Treatment Cysteamine (mercaptamine) n Cystagon (mercaptamine bitartrate) n


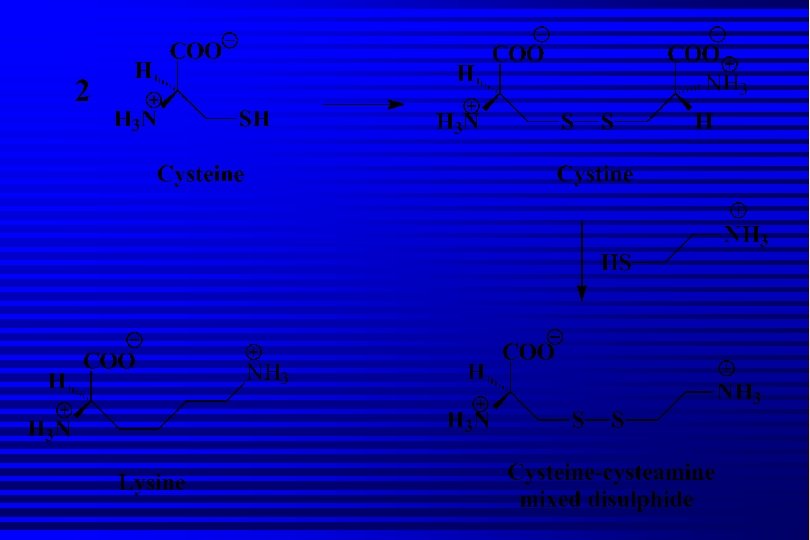
Cysteamine - problems Poor patient compliance due to offensive taste and smell n Excretion in breath and sweat leads to halitosis and body odour n Release in GI tract can cause nausea, vomiting, irritation etc. n Reformulate - sustained release, coated pellets n

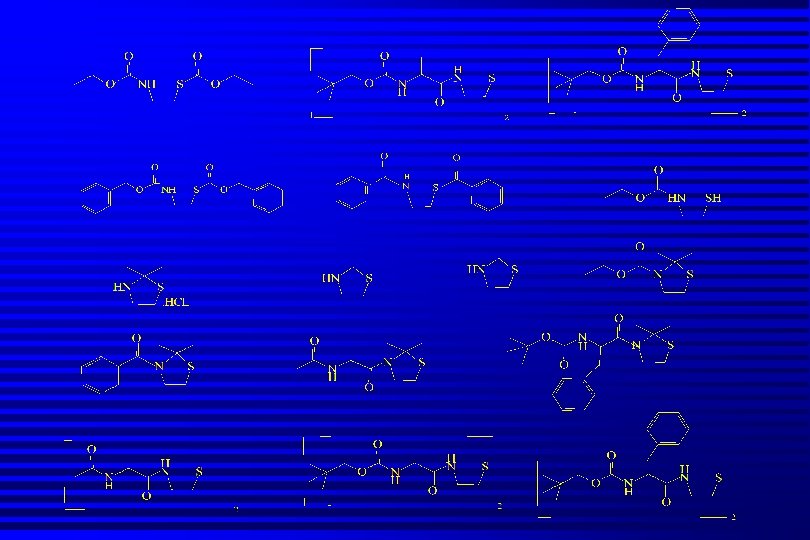
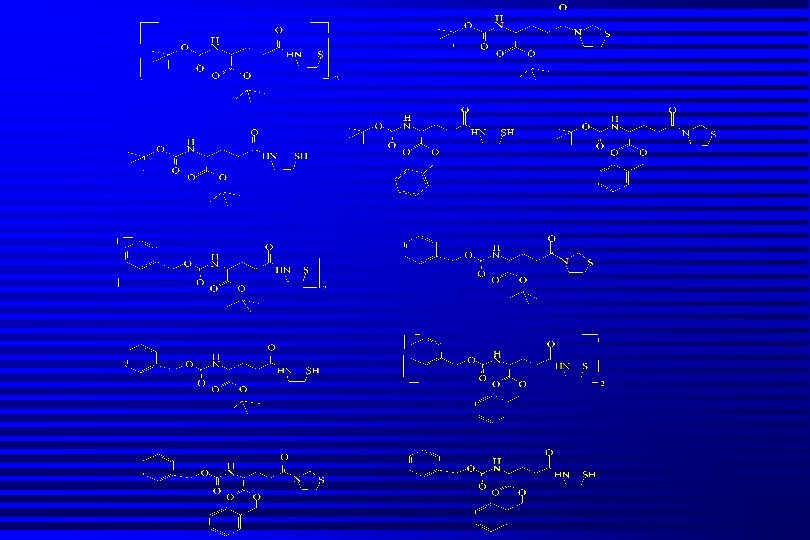
Pro-drugs of Cysteamine Pharmacologically inactive molecule metabolically activated in vivo to yield active compound. n Release of cysteamine is intra-cellular n Avoids GI related side-effects n Minimises odour problems n Increased lipophilicity improves uptake into cells, better PK and PD profiles n


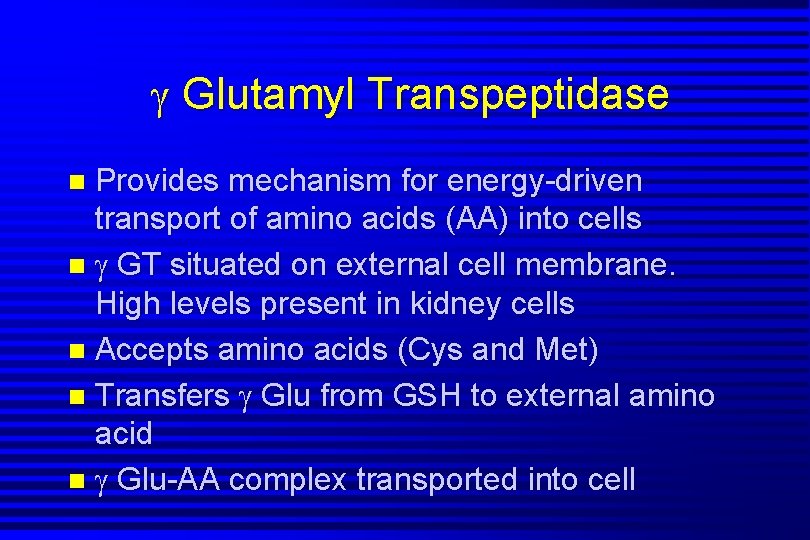
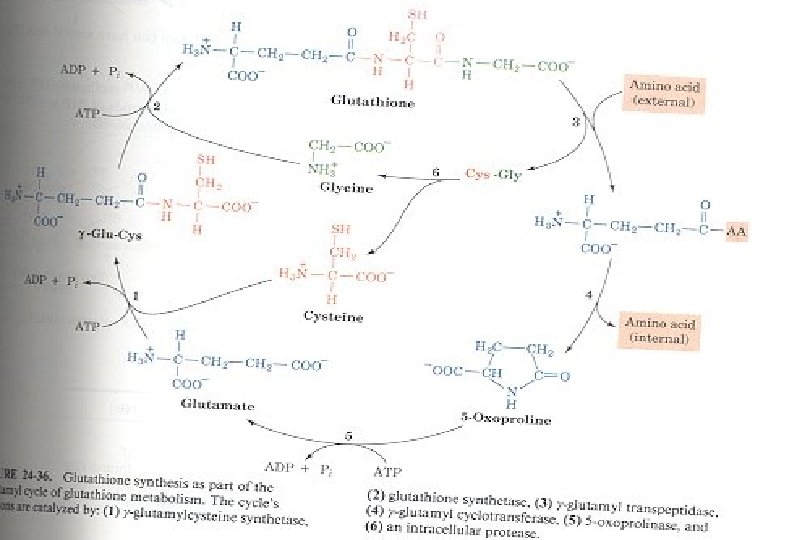
Glutamyl Transpeptidase Provides mechanism for energy-driven transport of amino acids (AA) into cells n GT situated on external cell membrane. High levels present in kidney cells n Accepts amino acids (Cys and Met) n Transfers Glu from GSH to external amino acid n Glu-AA complex transported into cell n



Cell culture Pro-drugs added to CHO Cell lines genetically modified to express GT. n Cytotoxicity and LD 50 determined (SRB) n Cells lysed with Triton X 100 and breakdown of compound followed by hplc-m/s n Histochemical detection of GT using L- L- glutamyl-p-nitroanilide and measurement of absorbance at 530 nm n


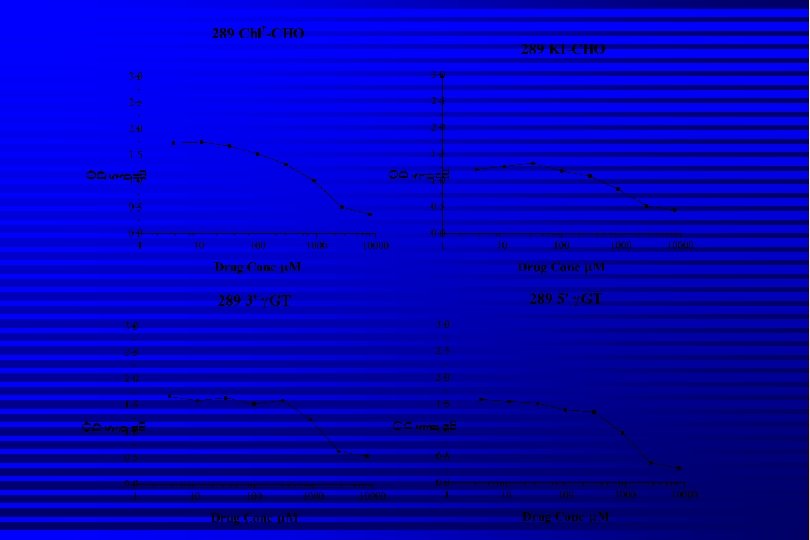
Results Pro-drugs synthesized in good yield and fully characterised spectroscopically n Pro-drugs taken up into CHO cells expressing -GT and detected by hplc n Levels of pro-drug fall wrt time but…. . n Unable to detect release of cysteamine using hplc-m/s n

Conclusions -glutamylcysteamine prodrugs can be readily synthesized in significant quantities n These prodrugs are almost entirely non-toxic to cells and are taken up successfully into CHO cells in vitro n The receptor enzyme -glutamyl transpeptidase is a valid vehicle for targetting cysteamine to the cells that most require it. n

Acknowledgements Wendy Cardwell and Dr Roz Anderson, University of Sunderland (synthesis) n Prof. Geoff Rowley and Dr Berwyn Owen, University of Sunderland (formulation) n Dr Malcolm Coulthard, RVI, Newcastle n Dr Andy Hall, University of Newcastle (cell culture) n Northern Region NHS for funding support n
