Descriptive Mineralogy Classification of Minerals Classification of the



























- Slides: 42

Descriptive Mineralogy Classification of Minerals

Classification of the Minerals • Non-Silicates – Native Elements – Halides – Sulfides – Oxides – Hydroxides – Carbonates – Sulfates – Phosphates • Silicates – Orthosilicates – Sorosilicates – Cyclosilicates – Chain Silicates – Layer Silicates – Tektosilicates

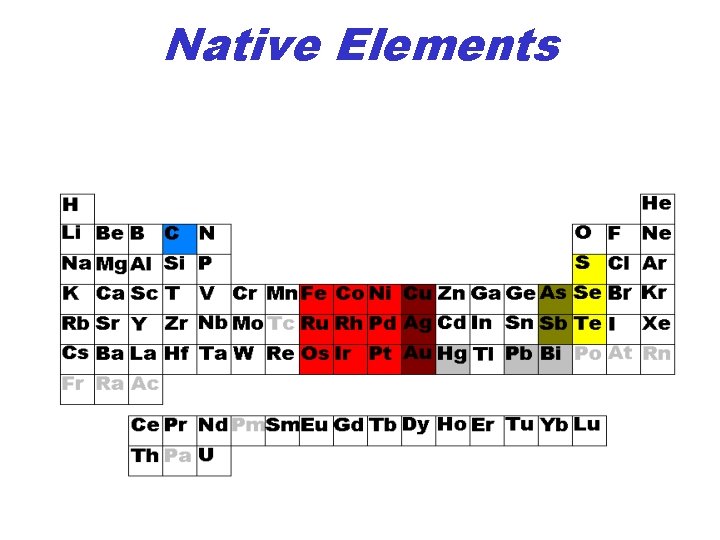
Native Elements

Native Elements: Metals • Fe, Co, Ni: Meteorites • Platinum Group: (Ru, Rh, Pd, Os, Ir, Pt): Mafic Igneous Rocks • Coinage metals: Cu, Ag, Au – Cu, Ag: Supergene Enrichments (Sulfide Oxidation) – Au: Low Temperature Hydrothermal, Placer

Native Elements: non-metals • Carbon: – Graphite: Metamorphic Rocks – Diamond: Kimberlites • Sulfur (+Se): – Salt Domes (Sulfate reduction), – Volcanos (H 2 S oxidation) • Tellurium (Te) Telluride oxidation

Halides: Minerals with halogen anions • Halite (Na. Cl) and Sylvite (KCl): Evaporites • Fluorite (Ca. F 2) Low temperature hydrothermal • Cryolite (Na 3 Al. F 6): Pegmatite

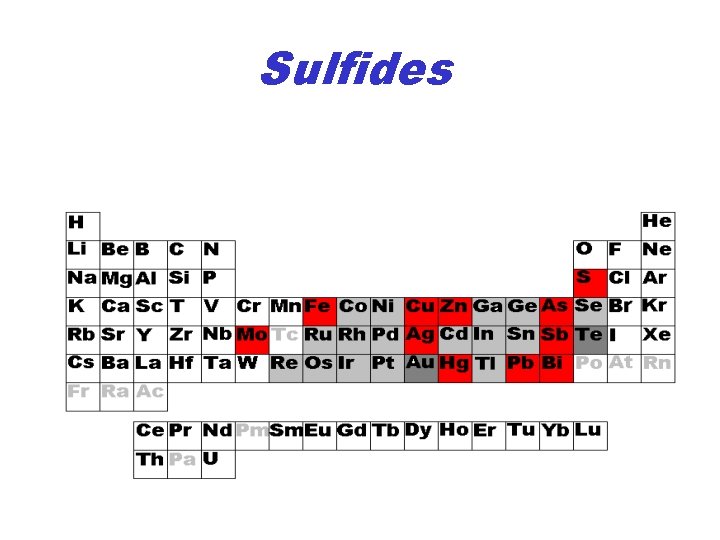
Sulfides

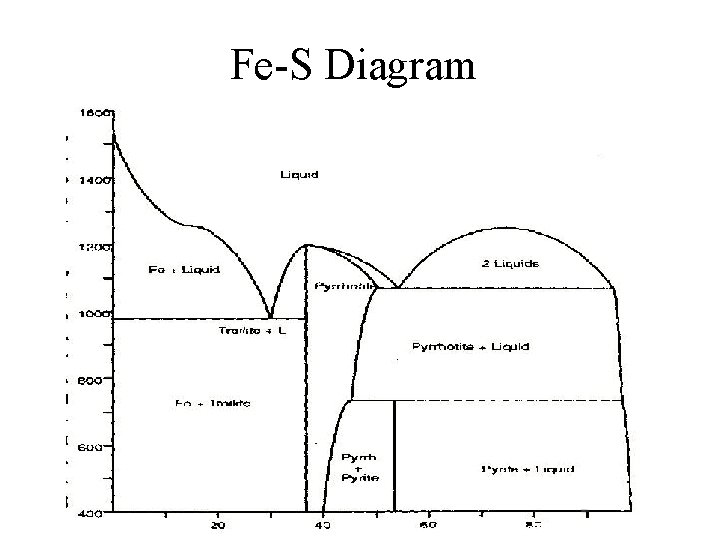
Fe Sulfides • Fe: – Pyrite Fe. S 2 – Marcasite Fe. S 2 – Pyrrhotite Fe 1 -x. S – Troilite Fe. S – Arsenopyrite Fe. As. S

Fe-S Diagram

Cu Sulfides • Cu Sulfides – Chalcocite Cu 2 S – Covellite Cu. S • Cu-Fe Sulfides – Chalcopyrite Cu. Fe. S 2 – Bornite Cu 5 Fe. S 4

Other Sulfides • • Sphalerite Zn. S Molybdenite Mo. S 2 Galena Pb. S Realgar As. S and Orpiment As 2 S 3 Arsenopyrite Fe. As. S Stibnite Sb 2 S 3 Cinnabar Hg. S

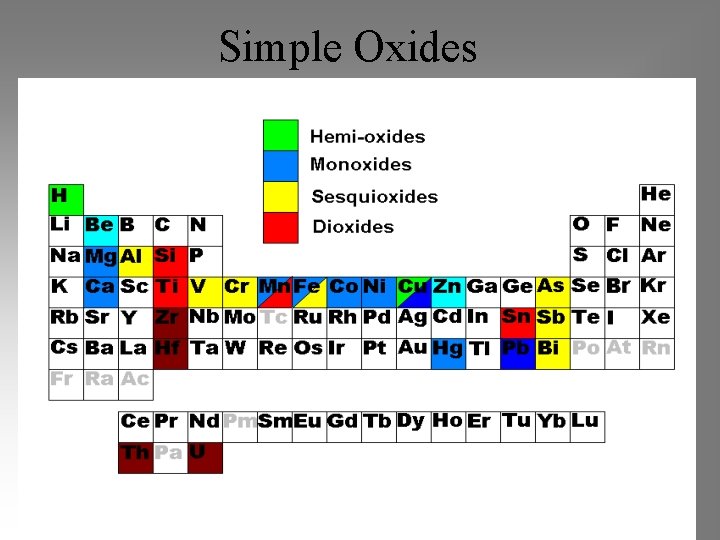
Simple Oxides • Hemioxides – Cuprite (Cu 2 O) – Ice (H 2 O) • Monoxides • Sesquioxides – Corundum (Al 2 O 3) – Hematite (Fe 2 O 3) – Bixbyite (Mn 2 O 3) • Dioxides – Periclase (Mg. O) – Rutile (Ti. O 2) – Wüstite (Fe. O) – Anatase (Ti. O 2) – Manganosite (Mn. O) – Brookite (Ti. O 2) – Lime (Ca. O) – Cassiterite(Sn. O 2) – Zincite (Zn. O) – Pyrolusite(Mn. O 2) – Bromellite (Be. O) – Tenorite (Cu. O)

Simple Oxides

Hemi-Oxides (M 2 O) • Ice (H 2 O) Hexagonal • Cuprite (Cu 2 O) • Why not Na 2 O? – (Na radius too large)

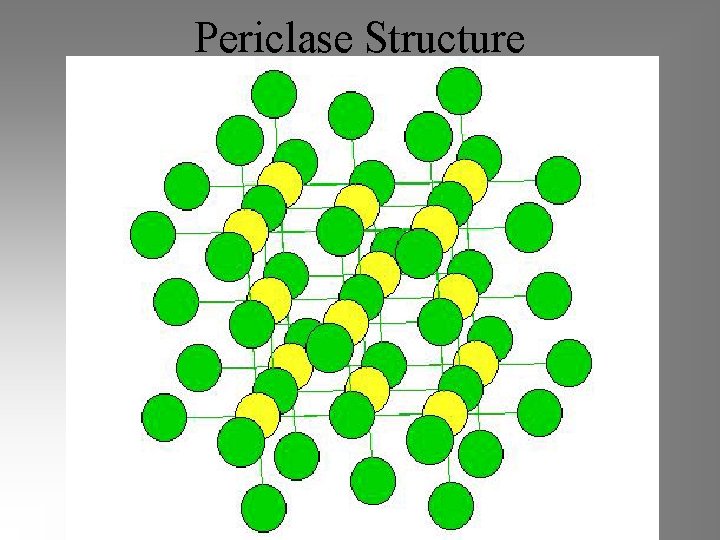
Monoxides (MO) • Rocksalt oxides Mg. O, Fe. O, Mn. O, Ca. O, Ni. O – Periclase Mg. O - Wuestite Fe. O – Manganosite Mn. O – Lime Ca. O – Bunsenite Ni. O • Zincite oxides: zincite Zn. O, bromellite Be. O • Other monoxides: – Tenorite Cu. O, Montroydite Hg. O

Periclase Structure

Sesquioxides (M 2 O 3) • Corundum Group – Corundum Al 2 O 3 – Hematite Fe 2 O 3 – Karelianite V 2 O 3 and Eskolaite Cr 2 O 3 • Other Sesquioxides – Bixbyite Mn 2 O 3

Dioxides • Rutile Group – Rutile Ti. O 2; Anatase; Brookite – Cassiterite Sn. O 2 – Pyrolusite Mn. O 2 – Stishovite Si. O 2 • Uraninite (UO 2) and Thorianite (Th. O 2) • Baddeleyite Zr. O 2

Complex Oxides • Two or more different cations – Spinel Group: M 2 TO 4 – Ilmenite Group : Fe. Ti. O 3 – Pseudobrookite Group : A 2 BO 5 – Perovskite Group : Ca. Ti. O 3 • High Pressure silicate analogues

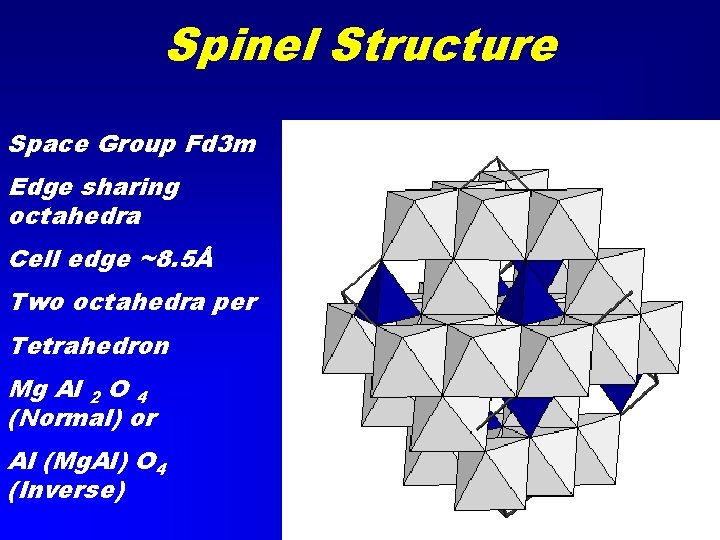
Spinel Group Spinel Mg. Al 2 O 4 Hercynite Fe. Al 2 O 4 Chromite Fe. Cr 2 O 4 Magnesiochromite Mg. Cr 2 O 4 • Magnetite Fe 2+Fe 3+2 O 4 • • • Magnesioferrite Mg. Fe 2 O 4 • Gahnite Zn. Al 2 O 4 • Ulvospinel Ti. Fe 2 O 4 • Ringwoodite Mg 2 Si. O 4

Spinel Structure Space Group Fd 3 m Edge sharing octahedra Cell edge ~8. 5Å Two octahedra per Tetrahedron Mg Al 2 O 4 (Normal) or Al (Mg. Al) O 4 (Inverse)

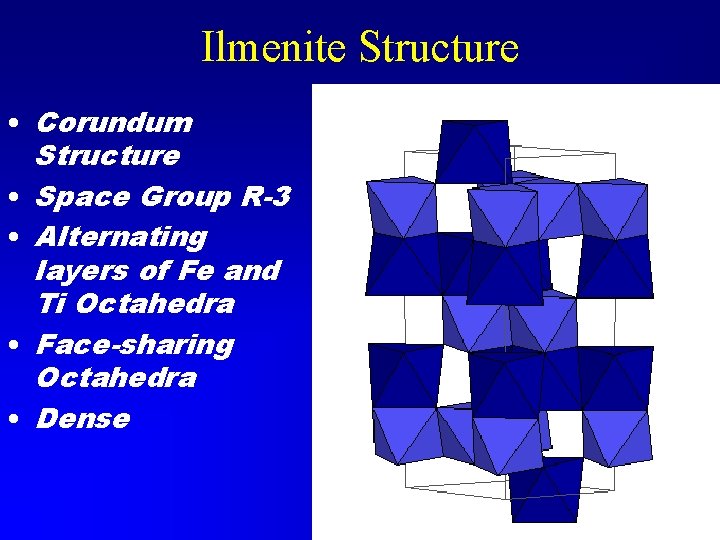
Ilmenite Group • Ilmenite Fe. Ti. O 3 • Geikielite Mg. Ti. O 3 • Akimotoite Mg. Si. O 3

Ilmenite Structure • Corundum Structure • Space Group R-3 • Alternating layers of Fe and Ti Octahedra • Face-sharing Octahedra • Dense

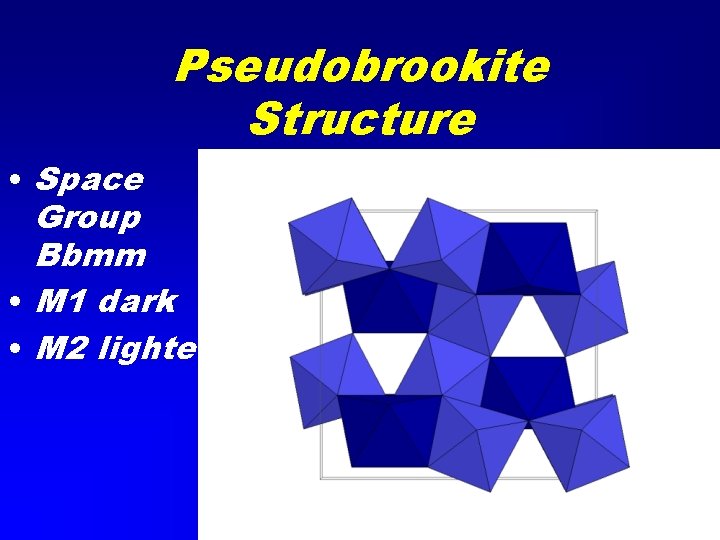
Pseudobrookite Group • Pseudobrookite Fe 2+Ti 2 O 5 • Ferro-pseudobrookite Ti. Fe 3+2 O 5 • Armalcolite (Mg, Fe 2+)Ti 2 O 5

Pseudobrookite Structure • Space Group Bbmm • M 1 dark • M 2 lighter

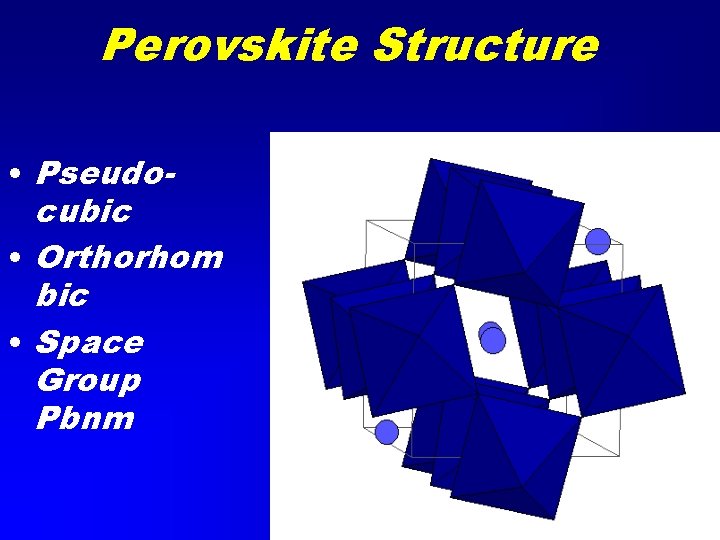
Perovskite • Perovskite Ca. Ti. O 3 • Mg. Si. O 3 (Lower mantle phase)

Perovskite Structure • Pseudocubic • Orthorhom bic • Space Group Pbnm

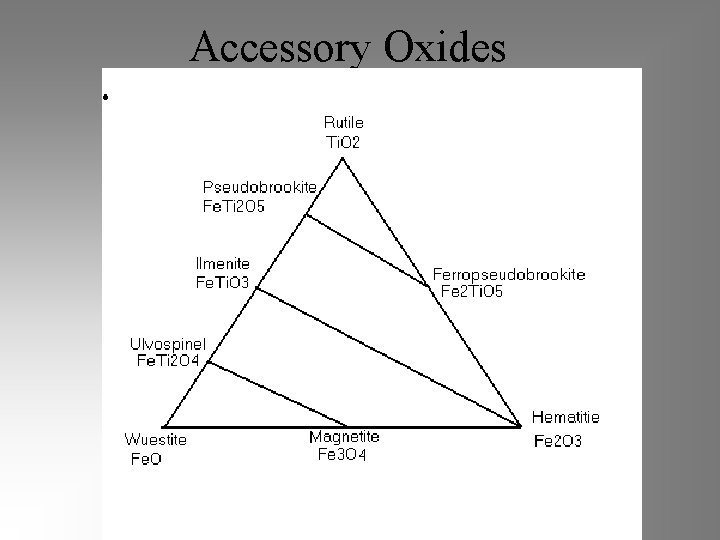
Accessory Oxides

Hydroxides Brucite Mg(OH)2 Gibbsite Al(OH)3 Boehmite and Diaspore Al. O(OH) Bauxite (Mixed Al hydroxides) Goethite Fe. O(OH) (+Lepidochrosite) • Limonite Fe(OH)3 • • •

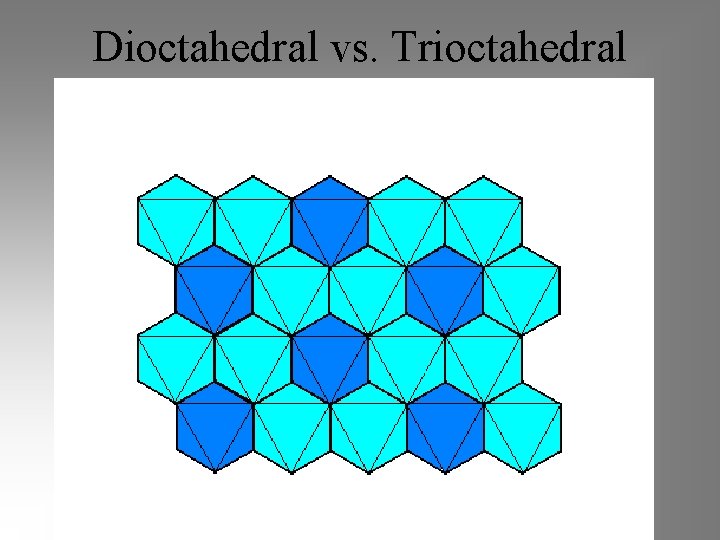
Dioctahedral vs. Trioctahedral

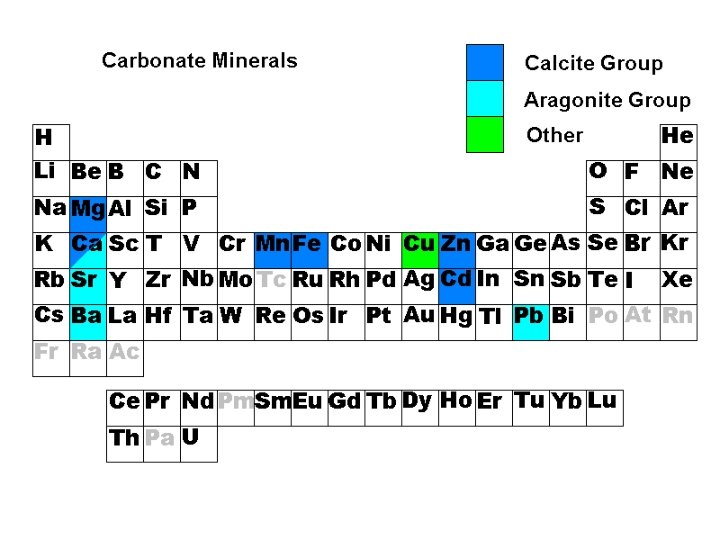
Carbonates • • Calcite Group Aragonite Group Dolomite Group Other carbonates


Calcite Group • • • Calcite Ca. CO 3 Magnesite Mg. CO 3 Siderite Fe. CO 3 Rhodochrosite Mn. CO 3 Smithsonite Zn. CO 3

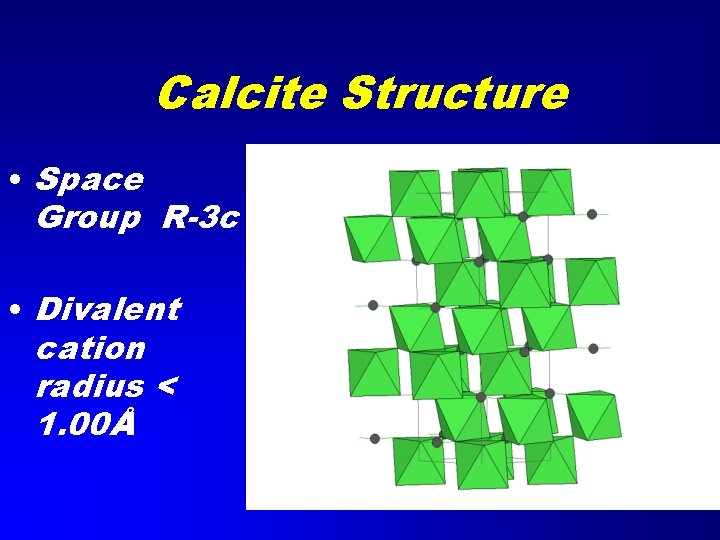
Calcite Structure • Space Group R-3 c • Divalent cation radius < 1. 00Å

Aragonite Group • Aragonite Ca. CO 3 • Strontianite Ca. CO 3 • Witherite Ba. CO 3 • Cerussite Pb. CO 3

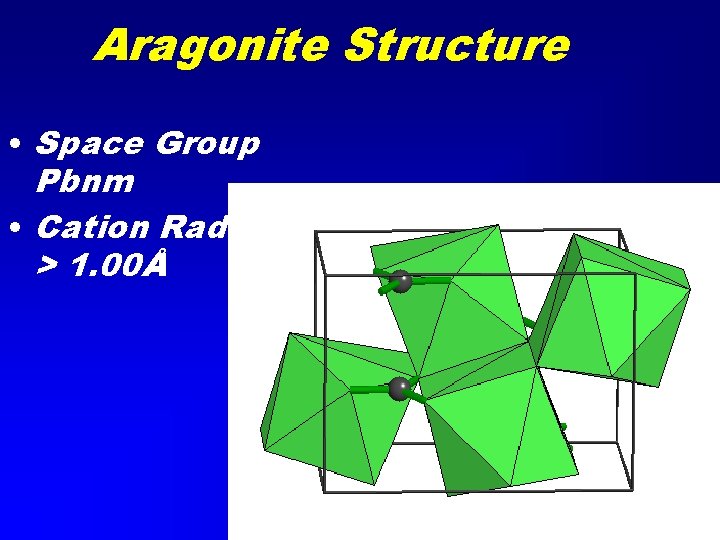
Aragonite Structure • Space Group Pbnm • Cation Radius > 1. 00Å

Dolomite Group • Dolomite Ca. Mg(CO 3)2 • Ankerite Ca. Fe(CO 3)2

Other Carbonates (+Nitrates) • Malachite (Green) Cu 2(OH)2 CO 3 • Azurite (Blue) Cu 3(OH)2(CO 3)2 – Cu 1+ or Cu 2+? • Bastnasite REECO 3 F • Niter KNO 3 • Soda Niter Na. NO 3

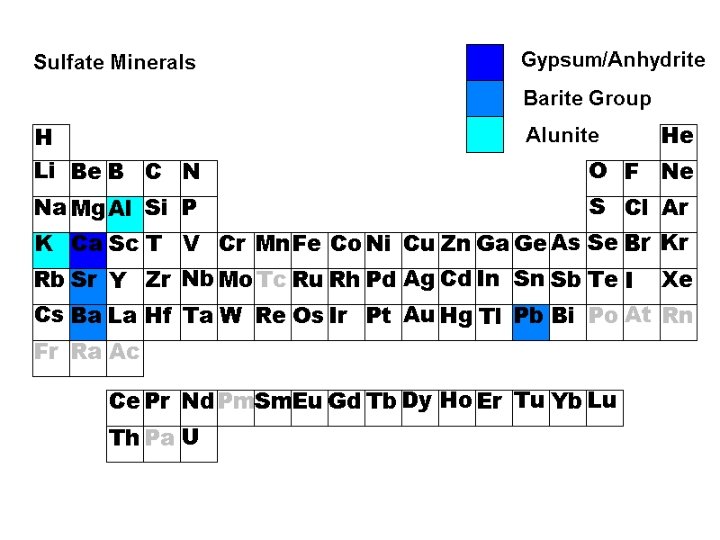
Sulfates • • • Gypsum Ca. SO 4 • 2 H 2 O Anhydrite Ca. SO 4 Celestine Sr. SO 4 Barite Ba. SO 4 Anglesite Pb. SO 4 Alunite KAl 3(OH)6(SO 4)2


Gypsum • Ca. SO 4 • 2 H 2 O • Hardness 2 • Evaporite Mineral • Contains molecular water Anhydrite • Ca. SO 4 • Hardness 3 -3. 5 • Evaporite Mineral • Contains no molecular water

Celestine • Sr. SO 4 Barite Ba. SO 4 • Hydrothermal • Anglesite Pb. SO 4 Ox. Hydrothermal