Describing Chemical Reactions Writing Chemical Equations In a

Describing Chemical Reactions

Writing Chemical Equations • In a chemical reaction, one or more substances (the reactants) change into one or more new substances (the products). • Chemists use a chemical equation – a quick, shorthand notation – to convey as much information as possible about what happens in a chemical reaction.

Word Equations • In shorthand for writing a description of a chemical reaction, the reactants are written on the left and the products on the right. • Reactants products • Iron + oxygen iron(III) oxide

Word Equations • To write a word equation, write the names of the reactants to the left of the arrow separated by plus signs; write the names of the products to the right of the arrow, also separated by plus signs.
Chemical Equations • A chemical equation is a representation of a chemical reaction. • Equations that show just the formulas of the reactants and products are called skeleton equations.
Chemical Equations • A skeleton equation is a chemical equation that does not indicate the relative amounts of the reactants and products. • Write the formulas of the reactants to the left of the yields sign (arrow) and the formulas of the products to the right.
Chemical Equations • To indicate the physical states of substances you can put a symbol after each formula: – (s) for solid – (l) for liquid – (g) for gas – (aq) for an aqueous solution • An aqueous solution is a substance dissolved in water.
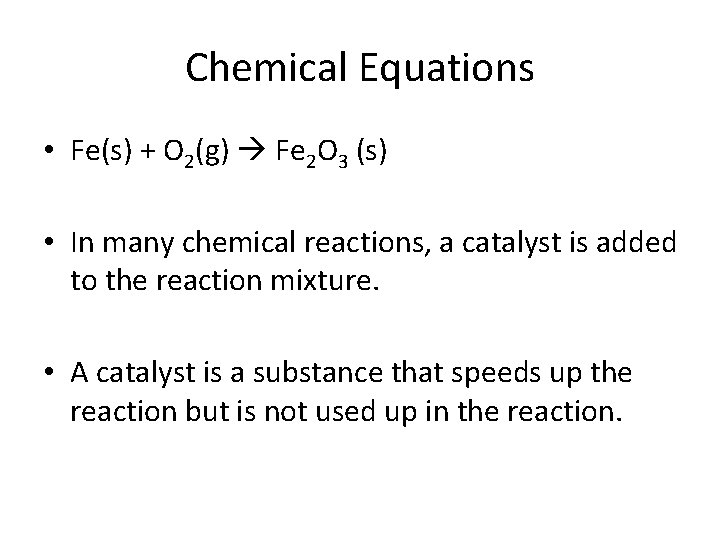
Chemical Equations • Fe(s) + O 2(g) Fe 2 O 3 (s) • In many chemical reactions, a catalyst is added to the reaction mixture. • A catalyst is a substance that speeds up the reaction but is not used up in the reaction.
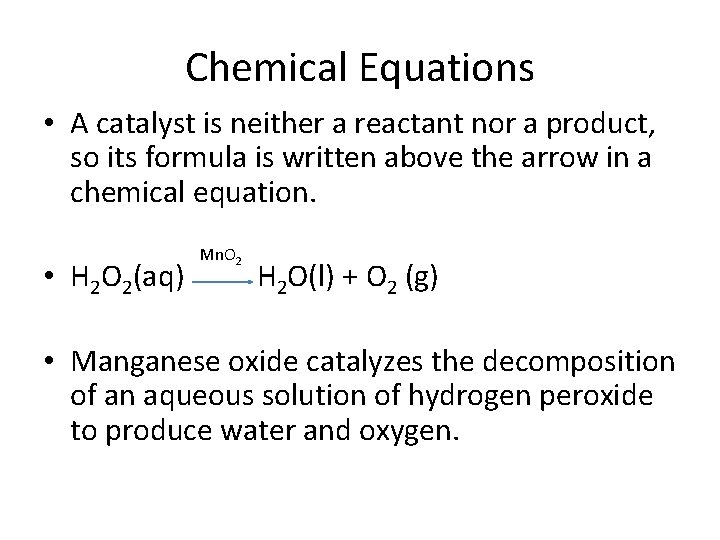
Chemical Equations • A catalyst is neither a reactant nor a product, so its formula is written above the arrow in a chemical equation. • H 2 O 2(aq) Mn. O 2 H 2 O(l) + O 2 (g) • Manganese oxide catalyzes the decomposition of an aqueous solution of hydrogen peroxide to produce water and oxygen.

Explaining and Writing Skeleton Equations • 1. write a sentence that describes this chemical reaction: – Na(s) + H 2 O(l) Na. OH(aq) + H 2(g)

Explaining and Writing Skeleton Equations • 2. sulfur burns in oxygen to form sulfur dioxide. Write a skeleton equation for this chemical reaction. Include appropriate physical state symbols.
Balancing Chemical Equations • An unbalanced equation does not indicate the quantity of the reactants needed to make the product. • To balance an equation you need to change the coefficients – small whole numbers that are placed in front of the formulas in an equation in order to balance it.
Balancing Chemical Equations • A chemical reaction is also described by a balanced equation in which each side of the equation has the same number of atoms of each element and mass is conserved. • The law of conservation of mass states that an equation must be balanced to show mass is conserved.
Balancing Chemical Equations • Representing a chemical reaction by a balanced chemical equation is a two-step process. • To write a complete chemical equation, write the skeleton equation. Then use coefficients to balance the equation so that it obeys the law of conservation of mass.
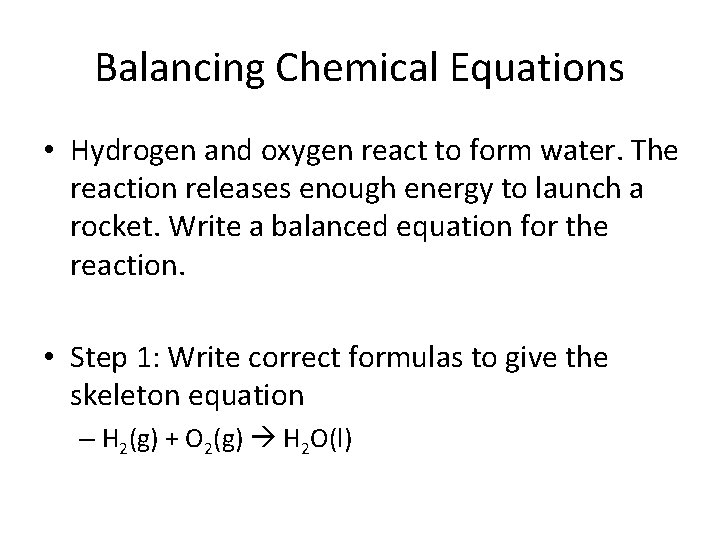
Balancing Chemical Equations • Hydrogen and oxygen react to form water. The reaction releases enough energy to launch a rocket. Write a balanced equation for the reaction. • Step 1: Write correct formulas to give the skeleton equation – H 2(g) + O 2(g) H 2 O(l)
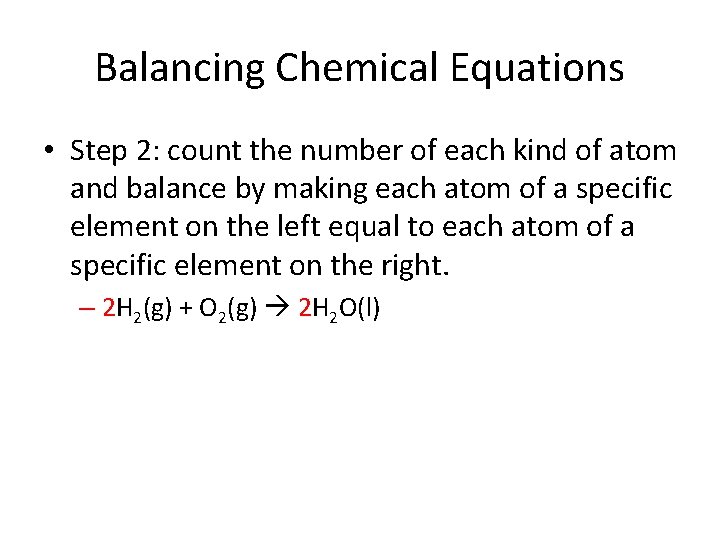
Balancing Chemical Equations • Step 2: count the number of each kind of atom and balance by making each atom of a specific element on the left equal to each atom of a specific element on the right. – 2 H 2(g) + O 2(g) 2 H 2 O(l)
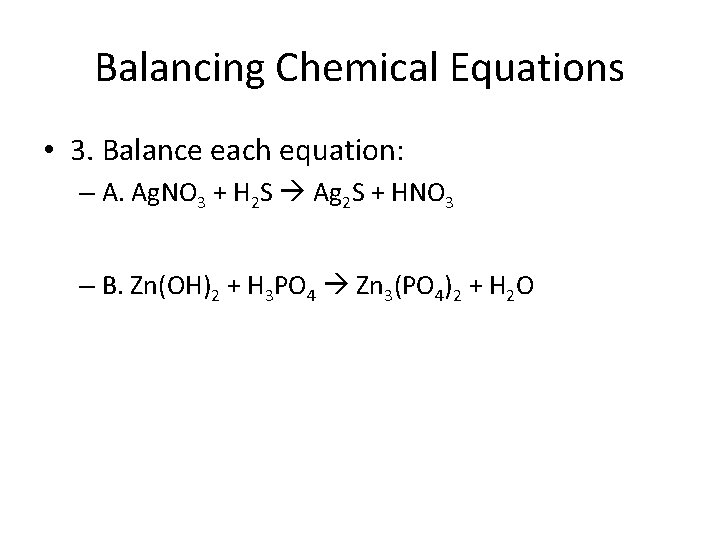
Balancing Chemical Equations • 3. Balance each equation: – A. Ag. NO 3 + H 2 S Ag 2 S + HNO 3 – B. Zn(OH)2 + H 3 PO 4 Zn 3(PO 4)2 + H 2 O
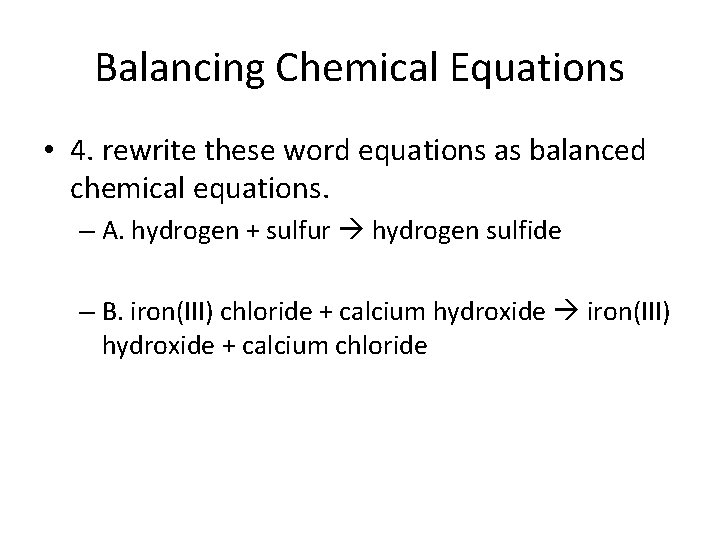
Balancing Chemical Equations • 4. rewrite these word equations as balanced chemical equations. – A. hydrogen + sulfur hydrogen sulfide – B. iron(III) chloride + calcium hydroxide iron(III) hydroxide + calcium chloride
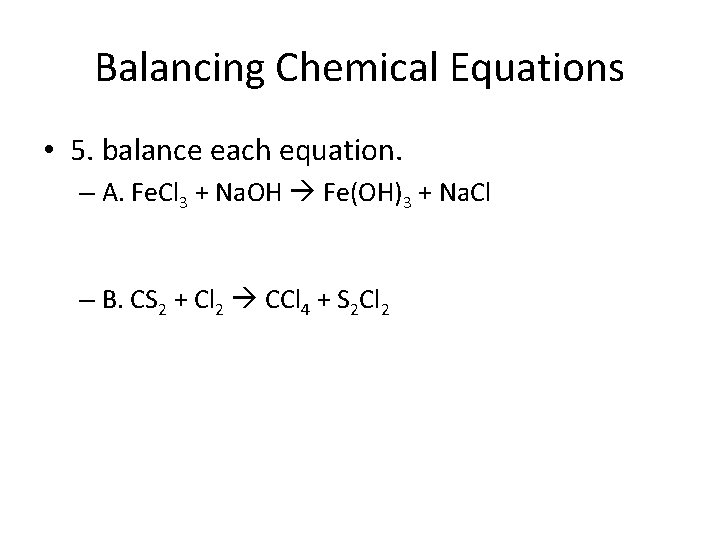
Balancing Chemical Equations • 5. balance each equation. – A. Fe. Cl 3 + Na. OH Fe(OH)3 + Na. Cl – B. CS 2 + Cl 2 CCl 4 + S 2 Cl 2
- Slides: 19