DESCRIBING CHEMICAL REACTIONS C 2 3 A chemical
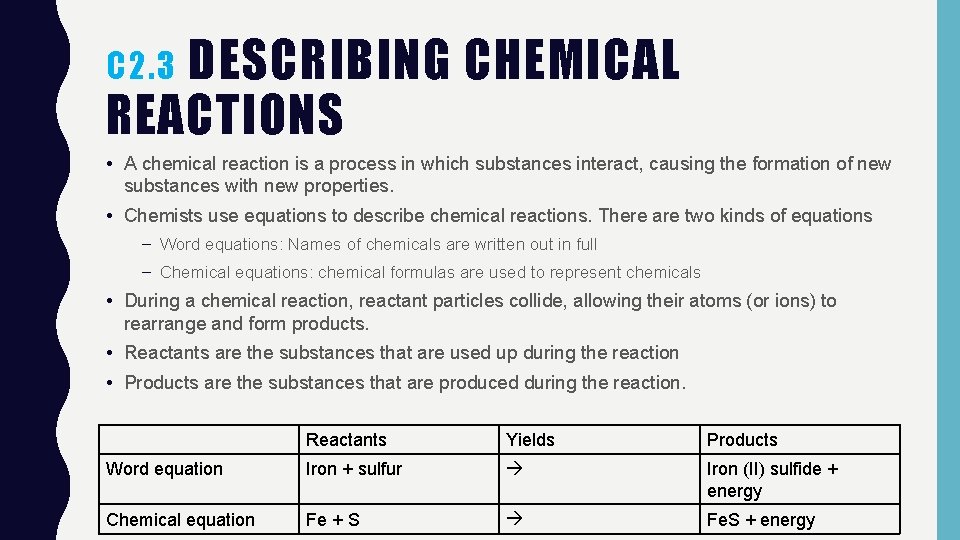
DESCRIBING CHEMICAL REACTIONS C 2. 3 • A chemical reaction is a process in which substances interact, causing the formation of new substances with new properties. • Chemists use equations to describe chemical reactions. There are two kinds of equations – Word equations: Names of chemicals are written out in full – Chemical equations: chemical formulas are used to represent chemicals • During a chemical reaction, reactant particles collide, allowing their atoms (or ions) to rearrange and form products. • Reactants are the substances that are used up during the reaction • Products are the substances that are produced during the reaction. Reactants Yields Products Word equation Iron + sulfur Iron (II) sulfide + energy Chemical equation Fe + S Fe. S + energy
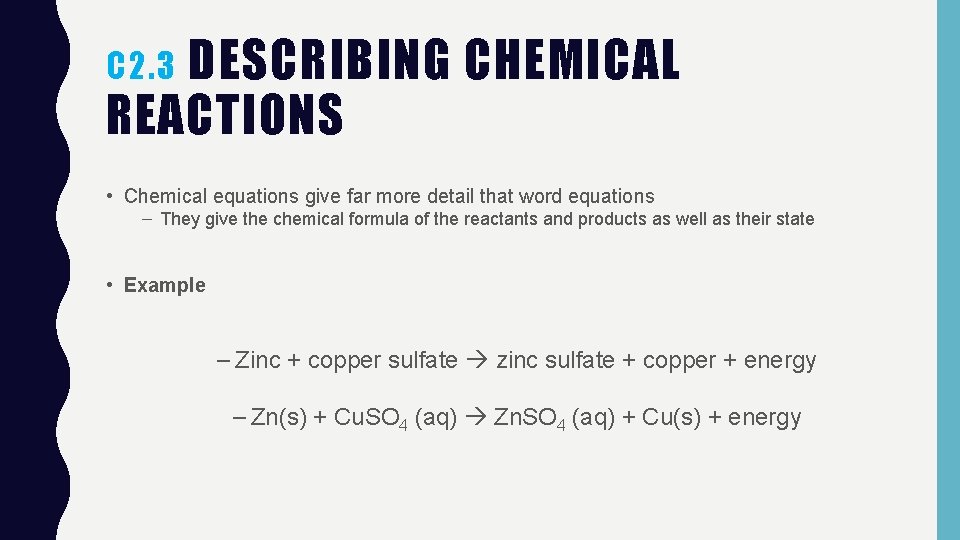
DESCRIBING CHEMICAL REACTIONS C 2. 3 • Chemical equations give far more detail that word equations – They give the chemical formula of the reactants and products as well as their state • Example – Zinc + copper sulfate zinc sulfate + copper + energy – Zn(s) + Cu. SO 4 (aq) Zn. SO 4 (aq) + Cu(s) + energy

C 3. 2 THE LAW OF CONSERVATION OF MASS AND ATOMIC THEORY • Law of conservation of mass: the statement that, in any given chemical reaction, the total mass of the reactants equals the total mass of the products. • Atoms cannot be created or destroyed, they are just rearranged to form products • All chemical equations must obey the law of conservation of mass – Read through explanation on p. 230 as a class (to show coefficients are added)
C 3. 4 BALANCING CHEMICAL EQUATIONS: ACTIVITY • Balance 7 of the 14 skeleton equations that are found on page 236 #7.
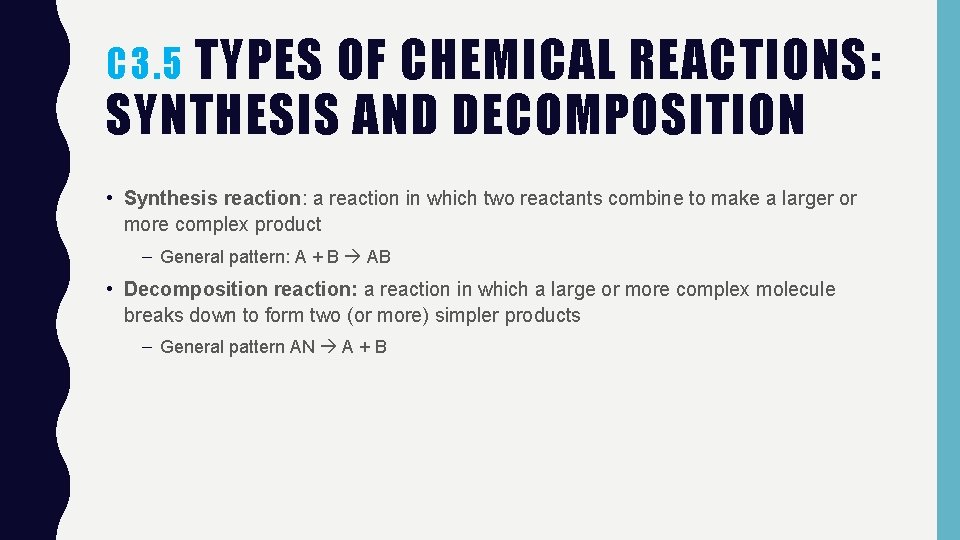
C 3. 5 TYPES OF CHEMICAL REACTIONS: SYNTHESIS AND DECOMPOSITION • Synthesis reaction: a reaction in which two reactants combine to make a larger or more complex product – General pattern: A + B AB • Decomposition reaction: a reaction in which a large or more complex molecule breaks down to form two (or more) simpler products – General pattern AN A + B
C 3. 5 TYPES OF CHEMICAL REACTIONS: SINGLE AND DOUBLE DISPLACEMENT • Single displacement reaction: A reaction in which an element displaces another element in a compound, producing a new compound a new element. – General pattern: A + BC AC + B • Double displacement reaction: A reaction that occurs when elements in different compounds displace each other or exchange places, producing two new compounds. – General Pattern: AB + CD AD + CB
- Slides: 6