Dermal Filler Medical Device Reporting MDR Amy C




- Slides: 11

Dermal Filler Medical Device Reporting (MDR) Amy C. Rogers, BSN, RN Office of Health Technology 4: Surgical and Infection Control Devices Office of Product Evaluation and Quality Center for Devices and Radiological Health U. S. Food and Drug Administration www. fda. gov 1

Medical Device Reports- Limitations • The MDR system provides FDA with timely information on medical device performance from patients, providers, and manufacturers. • While the MDR system is a valuable source of information, this passive surveillance system has limitations, including incomplete, inaccurate, untimely, unverified, or biased data in the reports. • In addition, the incidence or prevalence of an event cannot be determined from this reporting system alone due to potential underreporting, duplicate reporting of events, and the lack of information about the total number of devices. www. fda. gov 2

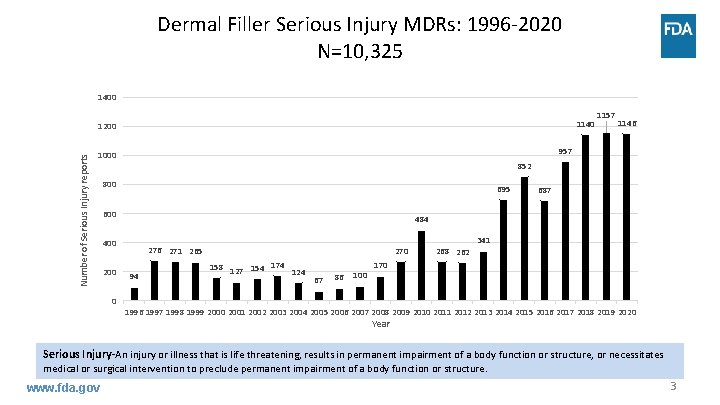
Dermal Filler Serious Injury MDRs: 1996 -2020 N=10, 325 1400 1140 Number of Serious Injury reports 1200 852 800 695 600 687 484 400 0 1146 957 1000 200 1157 276 271 265 94 270 158 174 127 154 124 67 86 100 268 262 341 170 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Year Serious Injury-An injury or illness that is life threatening, results in permanent impairment of a body function or structure, or necessitates medical or surgical intervention to preclude permanent impairment of a body function or structure. www. fda. gov 3

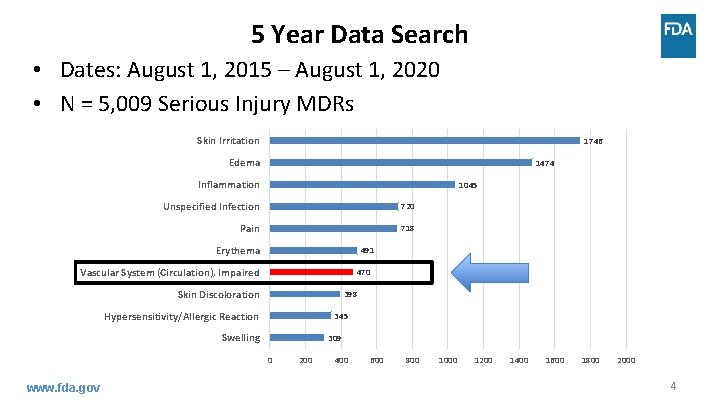
5 Year Data Search • Dates: August 1, 2015 – August 1, 2020 • N = 5, 009 Serious Injury MDRs Skin Irritation 1746 Edema 1474 Inflammation 1045 Unspecified Infection 720 Pain 718 Erythema 491 Vascular System (Circulation), Impaired 470 Skin Discoloration 398 Hypersensitivity/Allergic Reaction 345 Swelling 309 0 www. fda. gov 200 400 600 800 1000 1200 1400 1600 1800 2000 4

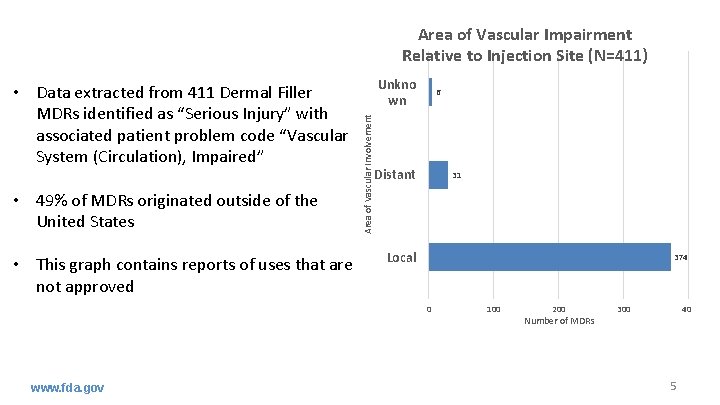
Area of Vascular Impairment Relative to Injection Site (N=411) • 49% of MDRs originated outside of the United States • This graph contains reports of uses that are not approved Unkno wn 6 Area of Vascular Involvement • Data extracted from 411 Dermal Filler MDRs identified as “Serious Injury” with associated patient problem code “Vascular System (Circulation), Impaired” Distant 31 Local 374 0 www. fda. gov 100 200 Number of MDRs 300 400 5

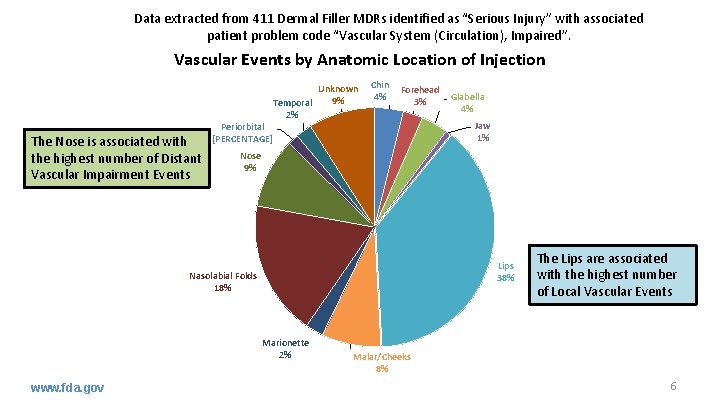
Data extracted from 411 Dermal Filler MDRs identified as “Serious Injury” with associated patient problem code “Vascular System (Circulation), Impaired”. Vascular Events by Anatomic Location of Injection Unknown 9% Temporal 2% The Nose is associated with the highest number of Distant Vascular Impairment Events Chin 4% Forehead 3% Periorbital [PERCENTAGE] Jaw 1% Nose 9% Lips 38% Nasolabial Folds 18% Marionette 2% www. fda. gov Glabella 4% The Lips are associated with the highest number of Local Vascular Events Malar/Cheeks 8% 6

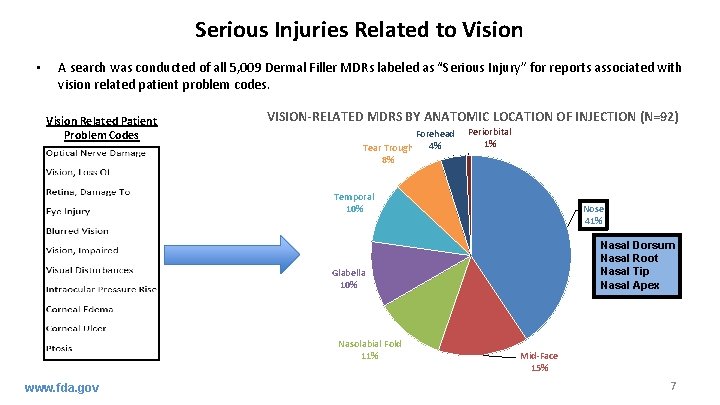
Serious Injuries Related to Vision • A search was conducted of all 5, 009 Dermal Filler MDRs labeled as “Serious Injury” for reports associated with vision related patient problem codes. Vision Related Patient Problem Codes VISION-RELATED MDRS BY ANATOMIC LOCATION OF INJECTION (N=92) Forehead 4% Tear Trough 8% Periorbital 1% Temporal 10% Nose 41% Nasal Dorsum Nasal Root Nasal Tip Nasal Apex Glabella 10% Nasolabial Fold 11% www. fda. gov Mid-Face 15% 7

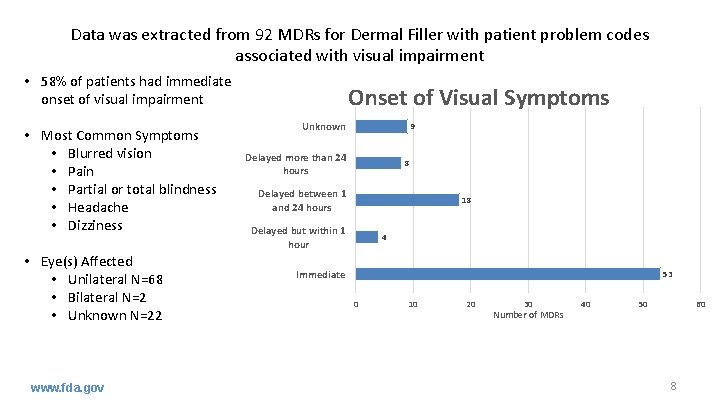
Data was extracted from 92 MDRs for Dermal Filler with patient problem codes associated with visual impairment • 58% of patients had immediate onset of visual impairment • Most Common Symptoms • Blurred vision • Pain • Partial or total blindness • Headache • Dizziness • Eye(s) Affected • Unilateral N=68 • Bilateral N=2 • Unknown N=22 www. fda. gov Onset of Visual Symptoms Unknown 9 Delayed more than 24 hours 8 Delayed between 1 and 24 hours 18 Delayed but within 1 hour 4 Immediate 53 0 10 20 30 Number of MDRs 40 50 60 8

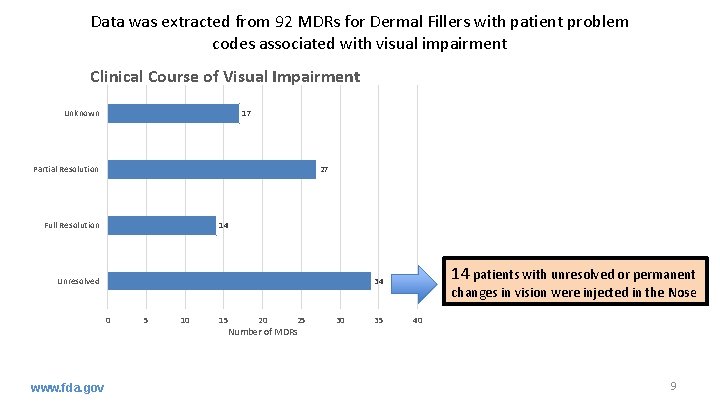
Data was extracted from 92 MDRs for Dermal Fillers with patient problem codes associated with visual impairment Clinical Course of Visual Impairment Unknown 17 Partial Resolution 27 Full Resolution 14 Unresolved 0 www. fda. gov 14 patients with unresolved or permanent 34 5 10 15 20 25 Number of MDRs 30 35 changes in vision were injected in the Nose 40 9

Of the 92 vision-related MDRs, there were 17 reports (17/92, 18. 5%) of patients who also experienced neurological symptoms. The most commonly reported symptoms • drowsiness • non-reactive pupil • absent light reflex • facial drooping • paralysis www. fda. gov 10

www. fda. gov 11