Deriving a netionic reaction Follow a stepbystep procedure

Deriving a net-ionic reaction

Follow a step-by-step procedure 1. Determine the molecular reaction. – Start with the formulae! – Balance the reaction. 2. Determine the solubility of the reactants and the products. – This tells you which species dissociate and which do not. 3. Write the total ionic reaction. – Identify spectator ions. 4. Write the net ionic reaction.
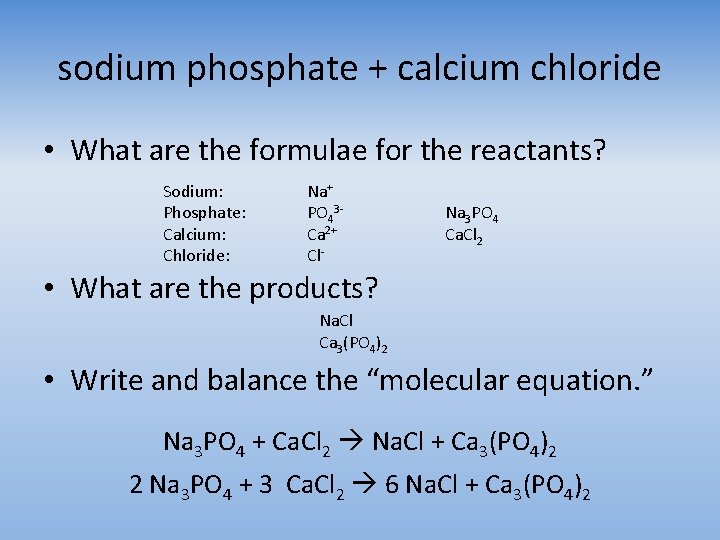
sodium phosphate + calcium chloride • What are the formulae for the reactants? Sodium: Phosphate: Calcium: Chloride: Na+ PO 43 Ca 2+ Cl- Na 3 PO 4 Ca. Cl 2 • What are the products? Na. Cl Ca 3(PO 4)2 • Write and balance the “molecular equation. ” Na 3 PO 4 + Ca. Cl 2 Na. Cl + Ca 3(PO 4)2 2 Na 3 PO 4 + 3 Ca. Cl 2 6 Na. Cl + Ca 3(PO 4)2

Use your Solubility Rules Na 3 PO 4 Ca. Cl 2 Na. Cl Ca 3(PO 4)2 soluble (sodium salts are soluble) soluble (chloride salts are soluble) soluble (see above!) insoluble (phosphate salts are insoluble) (except with group IA ions, such as Na+) Those salts that are soluble will dissociate into ions! Now, write the total ionic reaction.
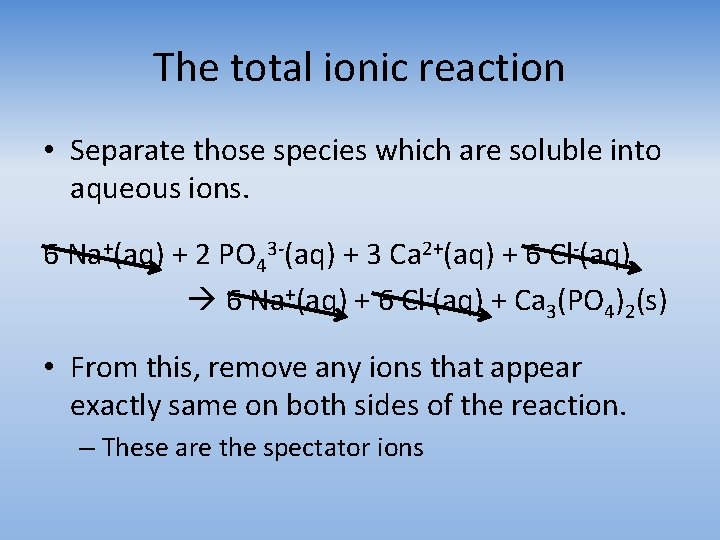
The total ionic reaction • Separate those species which are soluble into aqueous ions. 6 Na+(aq) + 2 PO 43 -(aq) + 3 Ca 2+(aq) + 6 Cl-(aq) 6 Na+(aq) + 6 Cl-(aq) + Ca 3(PO 4)2(s) • From this, remove any ions that appear exactly same on both sides of the reaction. – These are the spectator ions
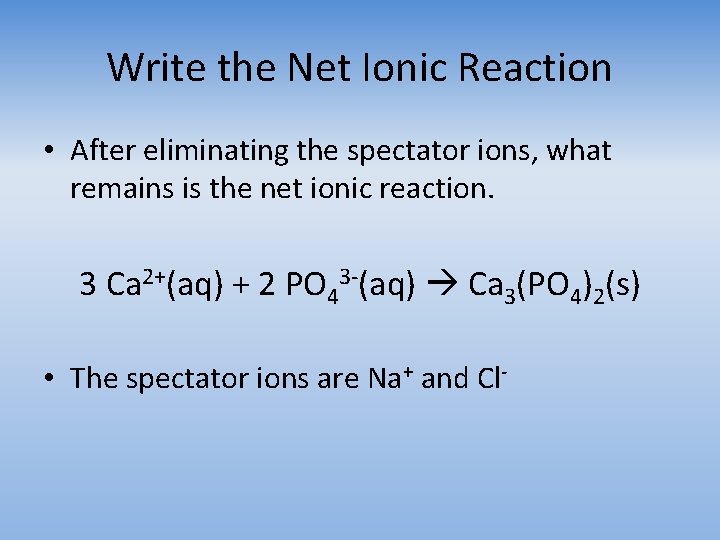
Write the Net Ionic Reaction • After eliminating the spectator ions, what remains is the net ionic reaction. 3 Ca 2+(aq) + 2 PO 43 -(aq) Ca 3(PO 4)2(s) • The spectator ions are Na+ and Cl-
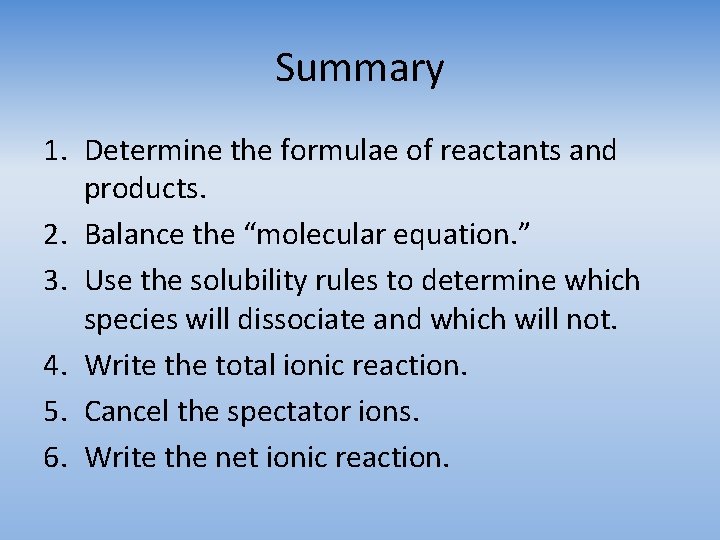
Summary 1. Determine the formulae of reactants and products. 2. Balance the “molecular equation. ” 3. Use the solubility rules to determine which species will dissociate and which will not. 4. Write the total ionic reaction. 5. Cancel the spectator ions. 6. Write the net ionic reaction.
- Slides: 7