Derivation of Nernst Equation for Single electrode Consider










- Slides: 21

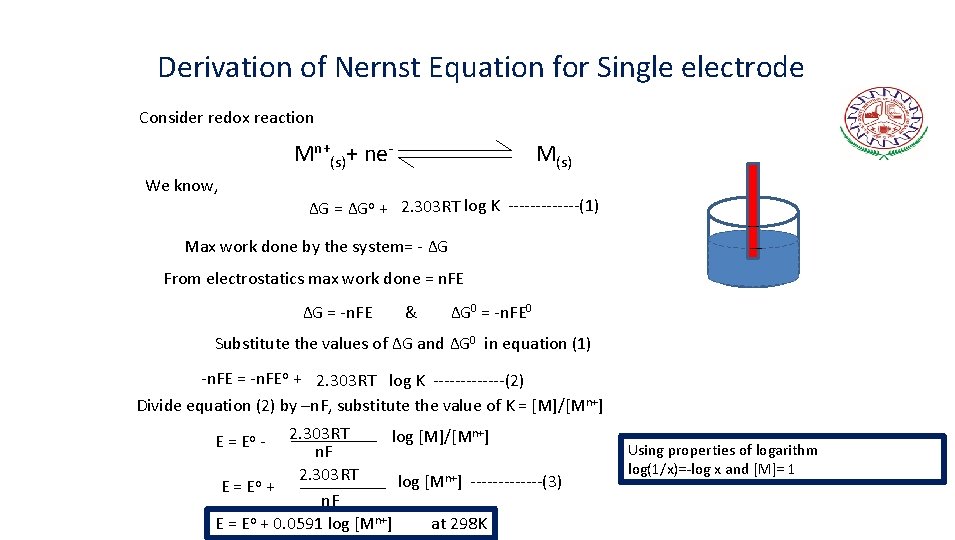
Derivation of Nernst Equation for Single electrode Consider redox reaction Mn+(s)+ ne. We know, M(s) ∆G = ∆Go + 2. 303 RT log K -------(1) Max work done by the system= - ∆G From electrostatics max work done = n. FE ∆G = -n. FE & ∆G 0 = -n. FE 0 Substitute the values of ∆G and ∆G 0 in equation (1) -n. FE = -n. FEo + 2. 303 RT log K -------(2) Divide equation (2) by –n. F, substitute the value of K = [M]/[Mn+] 2. 303 RT log [M]/[Mn+] n. F 2. 303 RT log [Mn+] -------(3) E = Eo + n. F E = Eo + 0. 0591 log [Mn+] at 298 K E = Eo - Using properties of logarithm log(1/x)=-log x and [M]= 1

Calomel and Silver-Silver Chloride Electrodes

Calomel electrode The calomel electrode is symbolically represented as, Pt (s) / Hg(l) / Hg 2 Cl 2(s) /Cl-

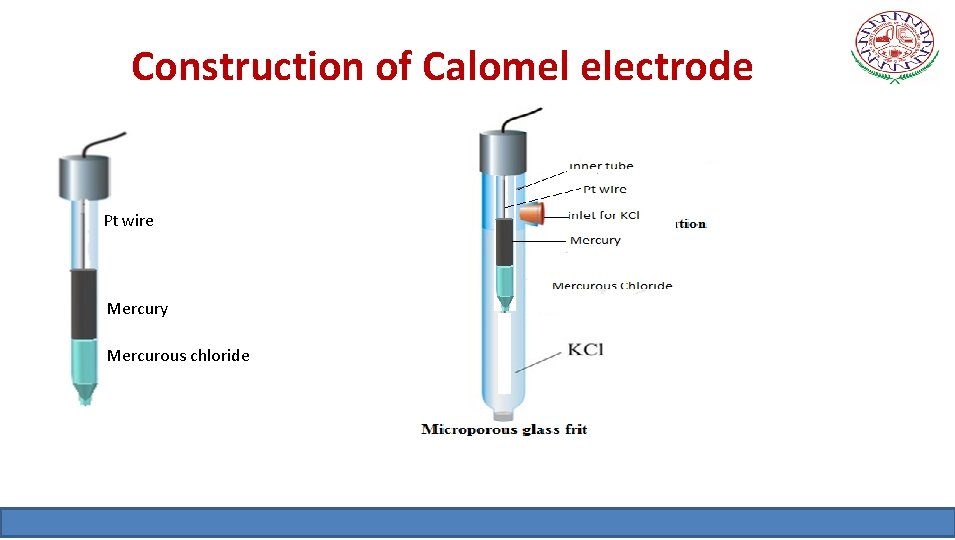
Construction of Calomel electrode Pt wire Mercury Mercurous chloride

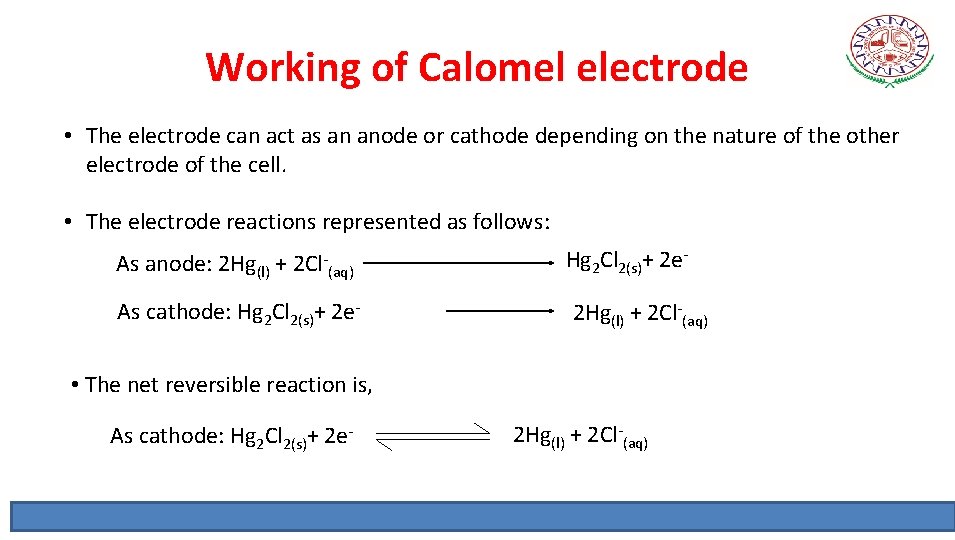
Working of Calomel electrode • The electrode can act as an anode or cathode depending on the nature of the other electrode of the cell. • The electrode reactions represented as follows: As anode: 2 Hg(l) + 2 Cl-(aq) Hg 2 Cl 2(s)+ 2 e- As cathode: Hg 2 Cl 2(s)+ 2 e- 2 Hg(l) + 2 Cl-(aq) • The net reversible reaction is, As cathode: Hg 2 Cl 2(s)+ 2 e- 2 Hg(l) + 2 Cl-(aq)

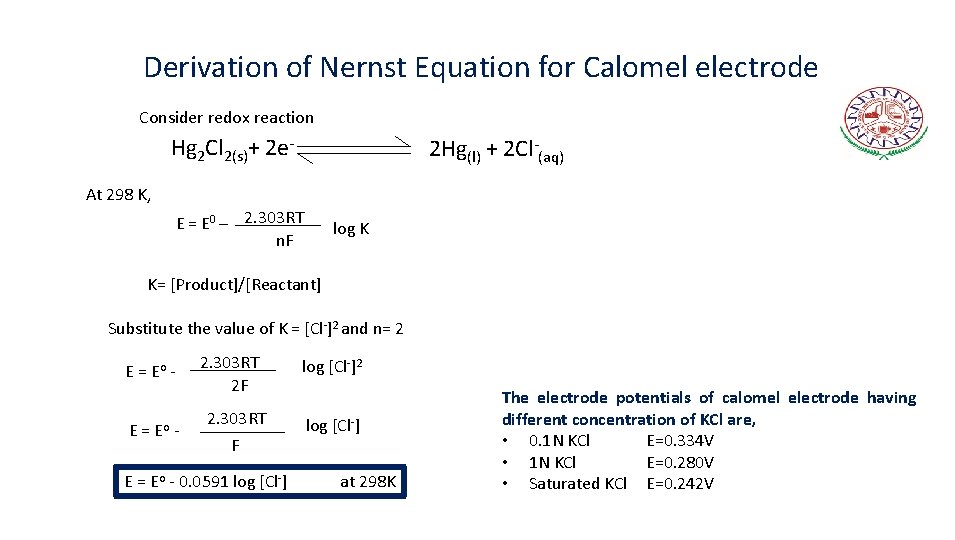
Derivation of Nernst Equation for Calomel electrode Consider redox reaction Hg 2 Cl 2(s)+ 2 e- 2 Hg(l) + 2 Cl-(aq) At 298 K, E = E 0 – 2. 303 RT n. F log K K= [Product]/[Reactant] Substitute the value of K = [Cl-]2 and n= 2 E = Eo - 2. 303 RT 2 F 2. 303 RT F E = Eo - 0. 0591 log [Cl-]2 log [Cl-] at 298 K The electrode potentials of calomel electrode having different concentration of KCl are, • 0. 1 N KCl E=0. 334 V • 1 N KCl E=0. 280 V • Saturated KCl E=0. 242 V

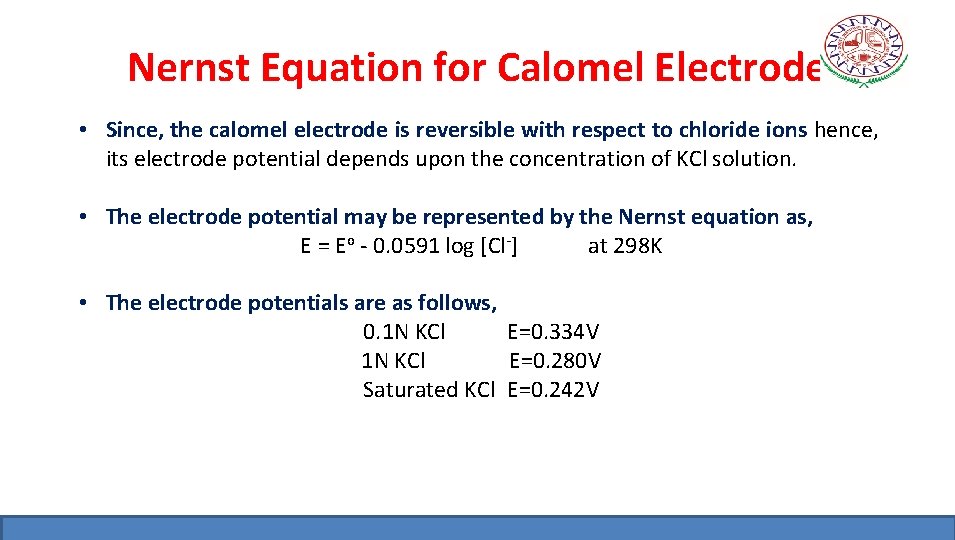
Nernst Equation for Calomel Electrode • Since, the calomel electrode is reversible with respect to chloride ions hence, its electrode potential depends upon the concentration of KCl solution. • The electrode potential may be represented by the Nernst equation as, E = Eo - 0. 0591 log [Cl-] at 298 K • The electrode potentials are as follows, 0. 1 N KCl E=0. 334 V 1 N KCl E=0. 280 V Saturated KCl E=0. 242 V

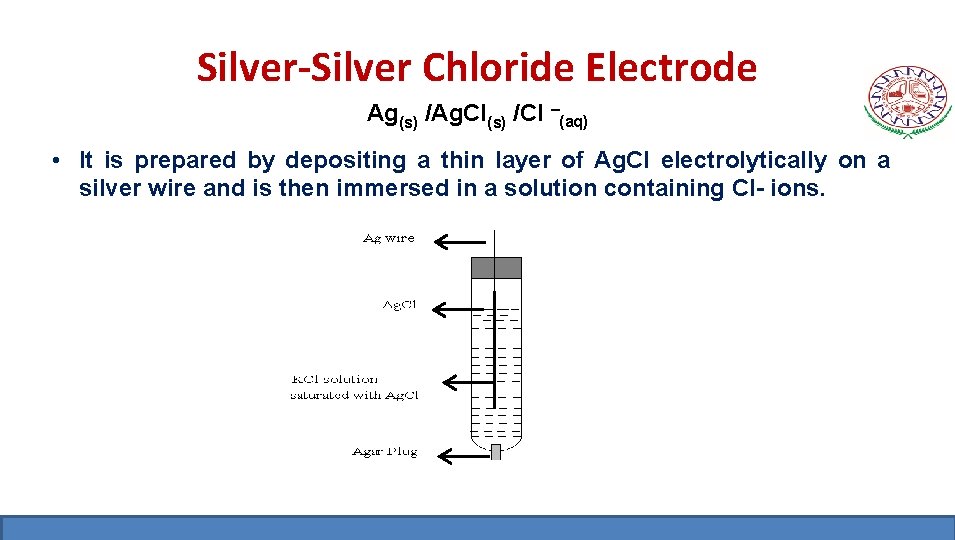
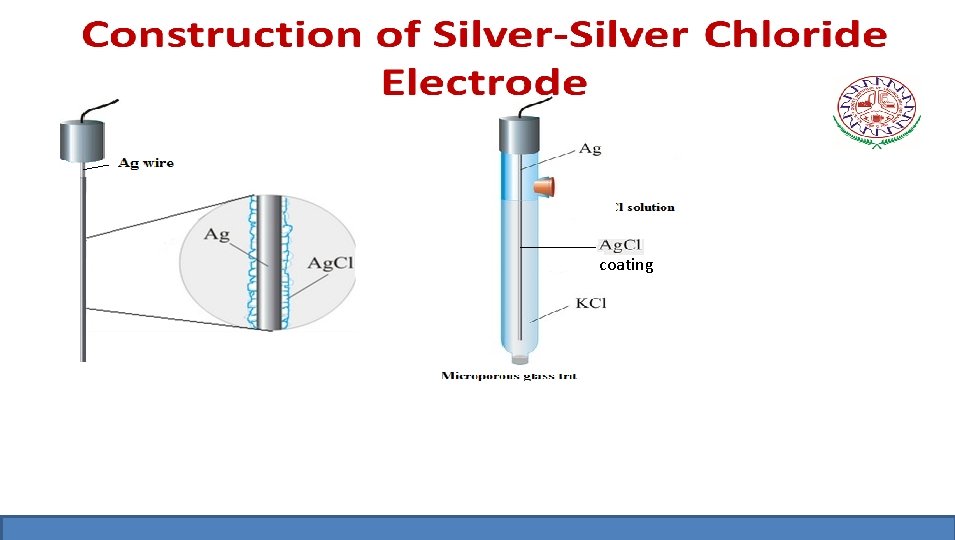
Silver-Silver Chloride Electrode Ag(s) /Ag. Cl(s) /Cl –(aq) • It is prepared by depositing a thin layer of Ag. Cl electrolytically on a silver wire and is then immersed in a solution containing Cl- ions.

coating

Working of Silver-Silver Chloride Electrode The electrode behaves both anode as well as cathode. The electrode reaction Ag(s) + Cl-(aq) Ag. Cl(s)+ e. The silver – silver chloride electrode is reversible with respect to chloride ions. Its electrode potential depends upon the concentration of KCl solution. Nernst equation: E = Eo - 0. 0591 log [Cl-] at 298 K For 0. 1 N KCl solution E= 0. 260 V For 1 N KCl solution E= 0. 223 V For Saturated KCl solution E = 0. 199 V at 298 K

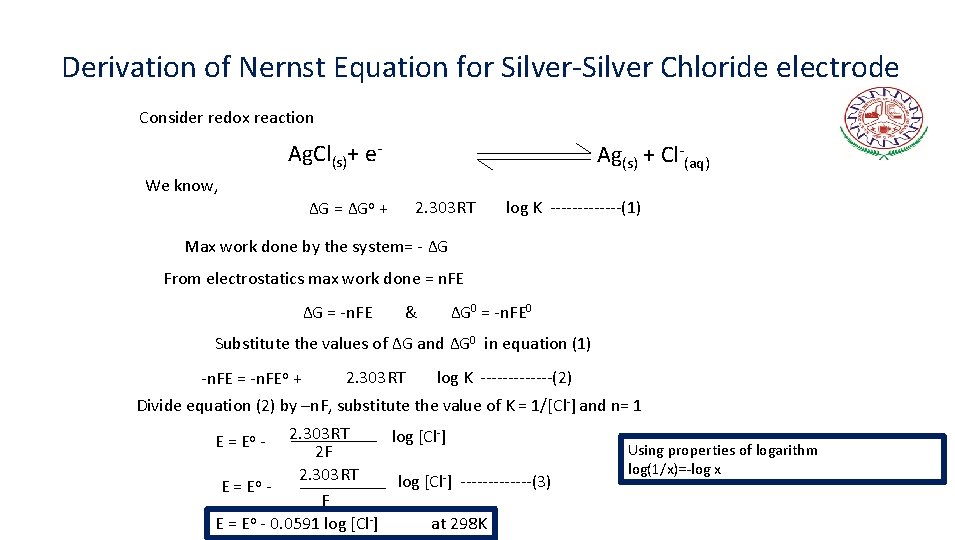
Derivation of Nernst Equation for Silver-Silver Chloride electrode Consider redox reaction Ag. Cl(s)+ e- Ag(s) + Cl-(aq) We know, 2. 303 RT ∆G = ∆Go + log K -------(1) Max work done by the system= - ∆G From electrostatics max work done = n. FE ∆G = -n. FE & ∆G 0 = -n. FE 0 Substitute the values of ∆G and ∆G 0 in equation (1) -n. FE = -n. FEo + 2. 303 RT log K -------(2) Divide equation (2) by –n. F, substitute the value of K = 1/[Cl-] and n= 1 2. 303 RT log [Cl-] 2 F 2. 303 RT log [Cl-] -------(3) E = Eo F E = Eo - 0. 0591 log [Cl-] at 298 K E = Eo - Using properties of logarithm log(1/x)=-log x

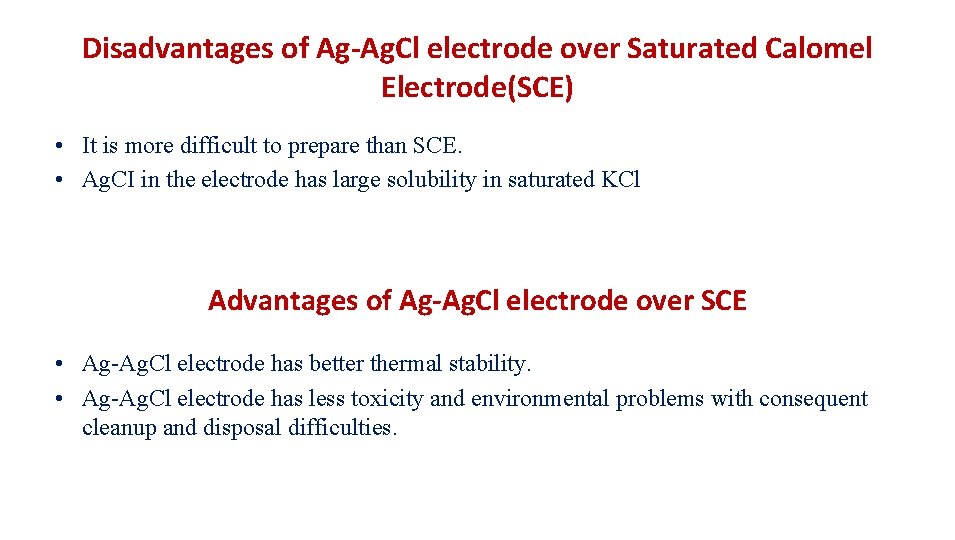
Disadvantages of Ag-Ag. Cl electrode over Saturated Calomel Electrode(SCE) • It is more difficult to prepare than SCE. • Ag. CI in the electrode has large solubility in saturated KCl Advantages of Ag-Ag. Cl electrode over SCE • Ag-Ag. Cl electrode has better thermal stability. • Ag-Ag. Cl electrode has less toxicity and environmental problems with consequent cleanup and disposal difficulties.

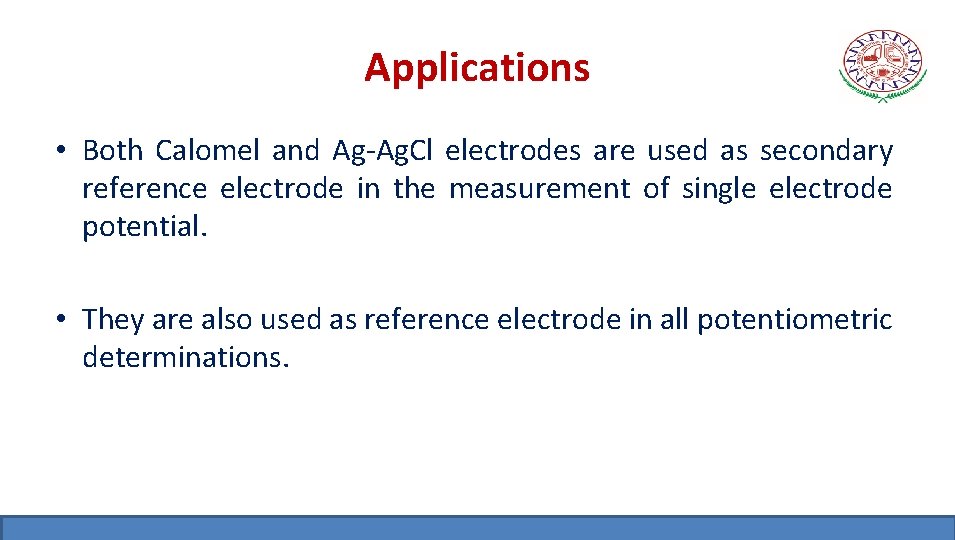
Applications • Both Calomel and Ag-Ag. Cl electrodes are used as secondary reference electrode in the measurement of single electrode potential. • They are also used as reference electrode in all potentiometric determinations.

Quiz 1. Calomel electrode is a a) Primary reference electrode b) Secondary reference electrode c) Metal – metal ion electrode d) Membrane electrode. 14

2. Calomel is a) Hg. Cl 2 c) KCl b) Ag. Cl d) Hg 2 Cl 2 15

3. Calomel electrode is reversible with respect to a) Mercuric ion b) Chloride ion c) Mercury d) Platinum 16

4. Ag-Ag. Cl electrode is a a) Primary reference electrode b) Secondary reference electrode c) metal – metal ion electrode d) membrane electrode 17

5. Ag – Ag. Cl electrode is a a) Metal – metal ion electrode b) Ion selective electrode c) Metal – insoluble salt electrode d) Gas electrode. 18

6. Silver-Silver chloride electrode is reversible with respect to a) Silver ion b) Chloride ion c) Mercury d) Platinum

7. Calomel electrode contains a) Saturated Hg. Cl 2 b) Saturated NH 4 Cl c) Saturated Ag. Cl d) Saturated Hg 2 Cl 2 20

8. Electrode potential of saturated Calomel electrode is a) 0. 242 V b) 0. 112 V c) 0. 420 V d) 0. 199 V 21