Department of OUTCOMES RESEARCH Clinical Research Design Types











- Slides: 20

Department of OUTCOMES RESEARCH

Clinical Research Design Types of Clinical Research Sources of Error Study Design Clinical Trials Daniel I. Sessler, M. D. Professor and Chair Department of OUTCOMES RESEARCH The Cleveland Clinic

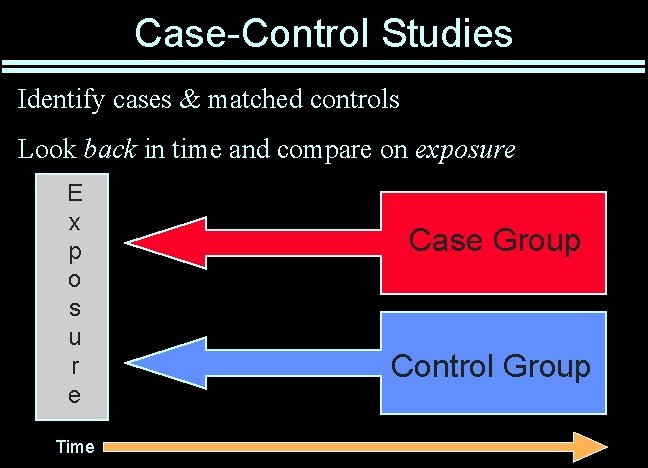
Case-Control Studies Identify cases & matched controls Look back in time and compare on exposure E x p o s u r e Time Case Group Control Group

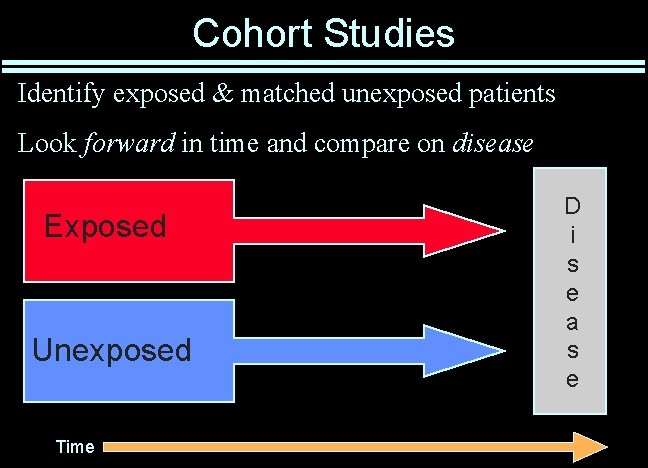
Cohort Studies Identify exposed & matched unexposed patients Look forward in time and compare on disease Exposed Unexposed Time D i s e a s e

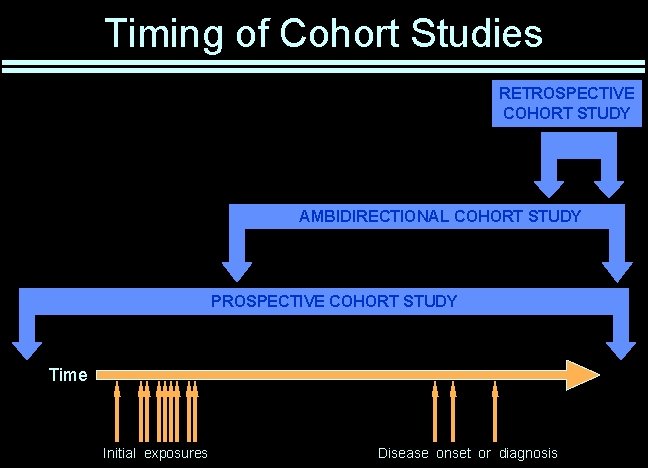
Timing of Cohort Studies RETROSPECTIVE COHORT STUDY AMBIDIRECTIONAL COHORT STUDY PROSPECTIVE COHORT STUDY Time Initial exposures Disease onset or diagnosis

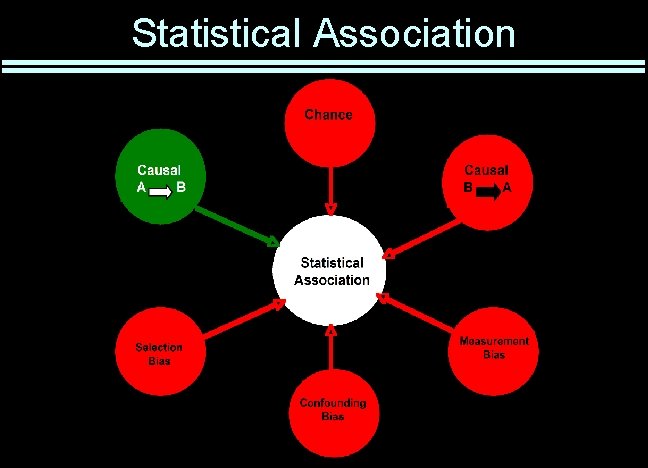
Sources of Error There is no perfect study • All are limited by practical and ethical considerations • It is impossible to control all potential confounders • Multiple studies required to prove a hypothesis Good design limits risk of false results • Statistics at best partially compensate for systematic error Major types of error • Selection bias • Measurement bias • Confounding • Reverse causation • Chance

Statistical Association

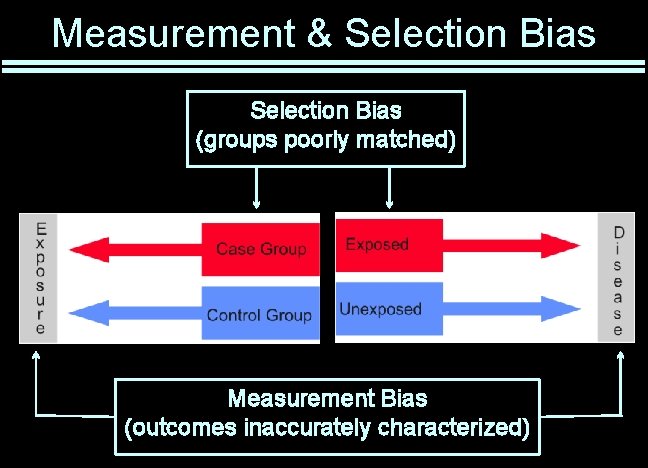
Selection Bias Non-random selection for inclusion / treatment • Or selective loss Subtle forms of disease may be missed When treatment is non-random: • Newer treatments assigned to patients most likely to benefit • “Better” patients seek out latest treatments • “Nice” patients may be given the preferred treatment Compliance may vary as a function of treatment • Patients drop out for lack of efficacy or because of side effects Largely prevented by randomization

Measurement Bias Quality of measurement varies non-randomly Quality of records generally poor • Not necessarily randomly so Patients given new treatments watched more closely Subjects with disease may better remember exposures When treatment is unblinded • Benefit may be over-estimated • Complications may be under-estimated Largely prevented by blinding

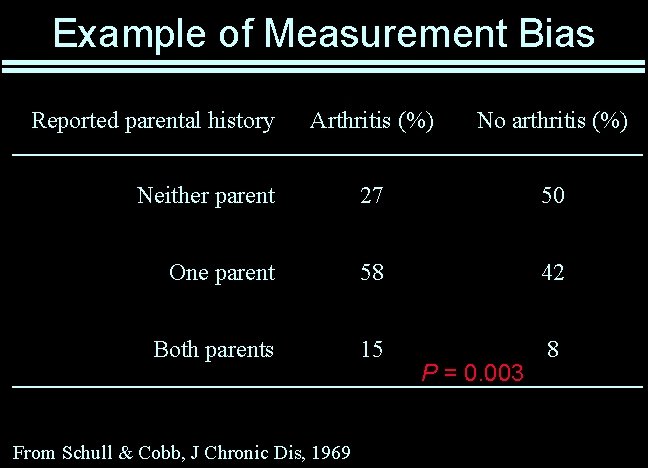
Example of Measurement Bias Reported parental history Arthritis (%) No arthritis (%) Neither parent 27 50 One parent 58 42 Both parents 15 8 From Schull & Cobb, J Chronic Dis, 1969 P = 0. 003

Measurement & Selection Bias (groups poorly matched) Measurement Bias (outcomes inaccurately characterized)

Confounding Association between two factors caused by third factor For example, mortality greater in Florida than Alaska • But average is much higher in Florida • Increased mortality results from age, rather than geography of FL For example, transfusions associated with mortality • But patients having larger, longer operations require more blood • Increased mortality may be a consequence of larger operations Largely prevented by randomization

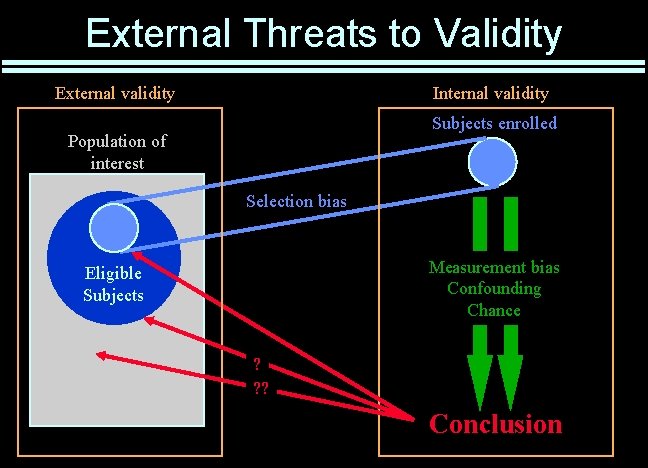
External Threats to Validity External validity Internal validity Subjects enrolled Population of interest Selection bias Measurement bias Confounding Chance Eligible Subjects ? ? ? Conclusion

Study Design Life is short; do things that matter! • Is the question important? Is it worth years of your life? Concise hypothesis testing of important outcomes • Usually one or two hypotheses per study • Beware of studies without a specified hypothesis A priori design • Planned comparisons with identified primary outcome • Intention-to-treat design Randomization • Best prevention for selection bias and confounding • Concealed allocation – No a priori knowledge of randomization

Subject Selection Appropriate for question • Maximize incidence of desired outcome • Technically, results apply only to population studied – Broaden enrollment to improve generalizability As restrictive as practical; trade-off between: • Variability • Ease of recruitment Sample-size estimates • Usually from preliminary data • Defined stopping points

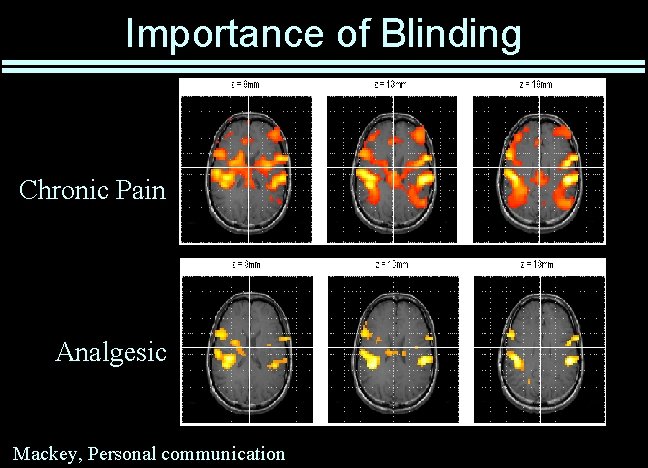
Blinding / Masking Only reliable way to prevent measurement bias • Essential for subjective responses – Use for objective responses whenever possible • Careful design required to maintain blinding Use double blinding whenever possible • Avoid triple blinding! Maintain blinding throughout data analysis • Even data-entry errors can be non-random • Statisticians are not immune to bias! Placebo effect can be enormous

Importance of Blinding Chronic Pain Analgesic Mackey, Personal communication

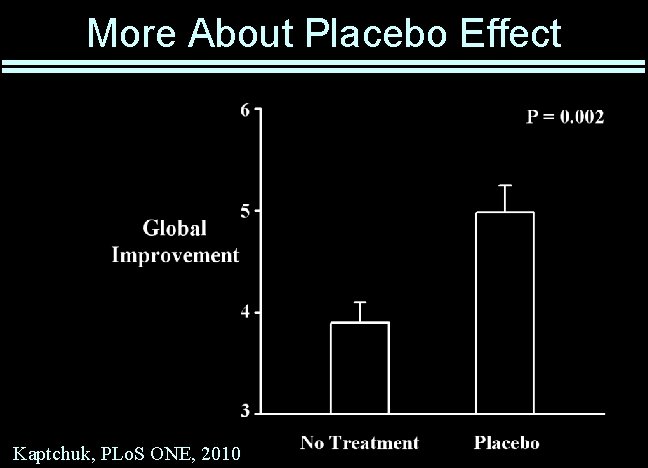
More About Placebo Effect Kaptchuk, PLo. S ONE, 2010

Conclusion: Good Clinical Studies… Test a specific a priori hypothesis • Evaluate clinically important outcomes Use appropriate statistical analysis Make conclusions that follow from the data • And acknowledged substantive limitations Randomize treatment & conceal allocation if possible • Best protection against selection bias and confounding Blind patients and investigators if possible • Best protection against measurement bias

Department of OUTCOMES RESEARCH