DEPARTMENT OF ORGANIC AND MACROMOLECULAR CHEMISTRY NMR AND











- Slides: 26

DEPARTMENT OF ORGANIC AND MACROMOLECULAR CHEMISTRY NMR AND STUCTURAL ANALYSIS RESEARCH GROUP CERAMIC CORE@SHELL NANOSPHERES AS VACCINE CARRIERS Livia Naszályi Nagy

VACCINES 2011 -2020 Decade of vaccines (WHO) Demand for new vaccines NANOVACCINES Targeted delivery may be achieved Simultaneous delivery of antigen and adjuvant NP elicits boosted immune response due to it’s size 2

Silica@Zirconia VACCINE CARRIERS In NMR 4 Nanos MIF project Nanoparticulate carriers with same surface chemistry – different size have to bind immune stimulator (adjuvant) for certain biological experiment have to be fluorescent be colloidally stable and do not release cargo at p. H 7. 4 Immune stimulator: Cp. G ODN 1826 (phosphorothioate backbone) 5’-tccatgacgttcctgacgtt-3’ silica zirconia immune stimulator 3

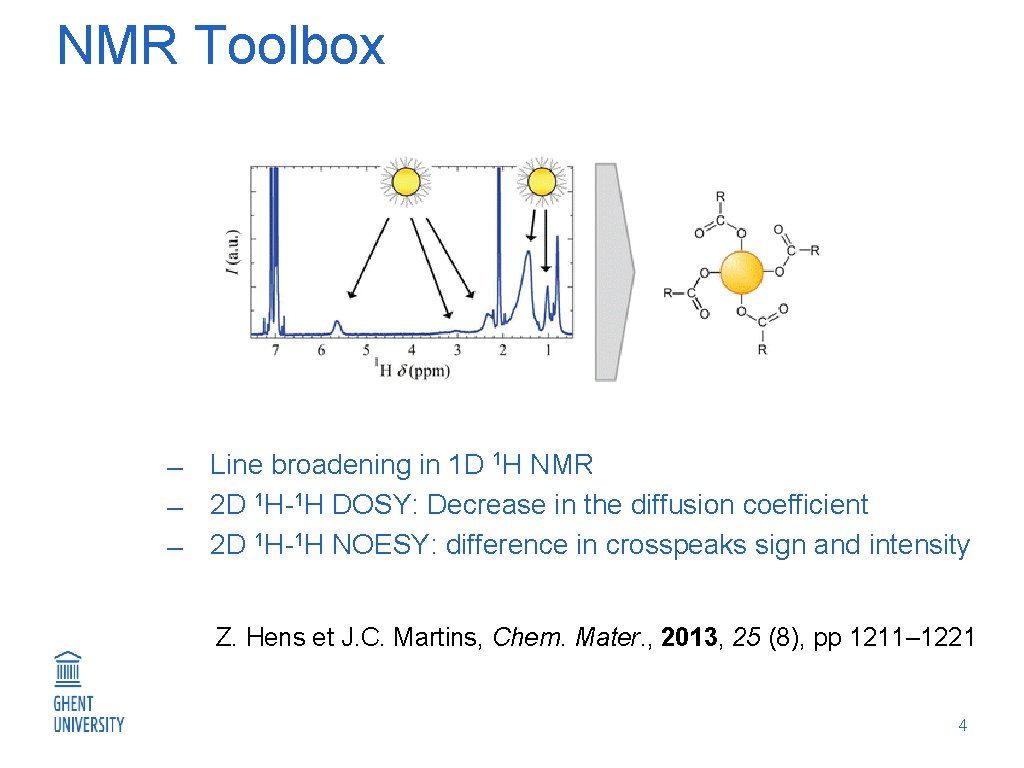
NMR Toolbox Line broadening in 1 D 1 H NMR 2 D 1 H-1 H DOSY: Decrease in the diffusion coefficient 2 D 1 H-1 H NOESY: difference in crosspeaks sign and intensity Z. Hens et J. C. Martins, Chem. Mater. , 2013, 25 (8), pp 1211– 1221 4

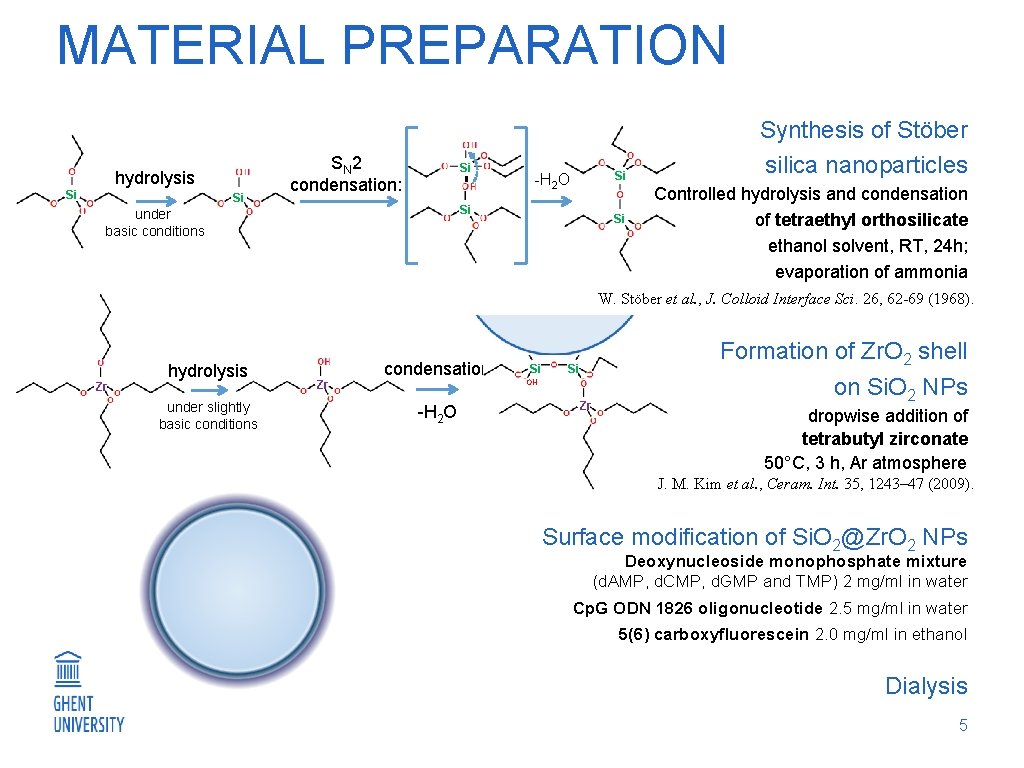
MATERIAL PREPARATION hydrolysis Si Si SN 2 condensation: Si Si -H 2 O Si under basic conditions Si Synthesis of Stöber silica nanoparticles Controlled hydrolysis and condensation of tetraethyl orthosilicate ethanol solvent, RT, 24 h; evaporation of ammonia W. Stöber et al. , J. Colloid Interface Sci. 26, 62 -69 (1968). Zr hydrolysis under slightly basic conditions Zr condensation -H 2 O Si Si OH Zr Formation of Zr. O 2 shell on Si. O 2 NPs dropwise addition of tetrabutyl zirconate 50°C, 3 h, Ar atmosphere J. M. Kim et al. , Ceram. Int. 35, 1243– 47 (2009). Surface modification of Si. O 2@Zr. O 2 NPs Deoxynucleoside monophosphate mixture (d. AMP, d. CMP, d. GMP and TMP) 2 mg/ml in water Cp. G ODN 1826 oligonucleotide 2. 5 mg/ml in water 5(6) carboxyfluorescein 2. 0 mg/ml in ethanol Dialysis 5

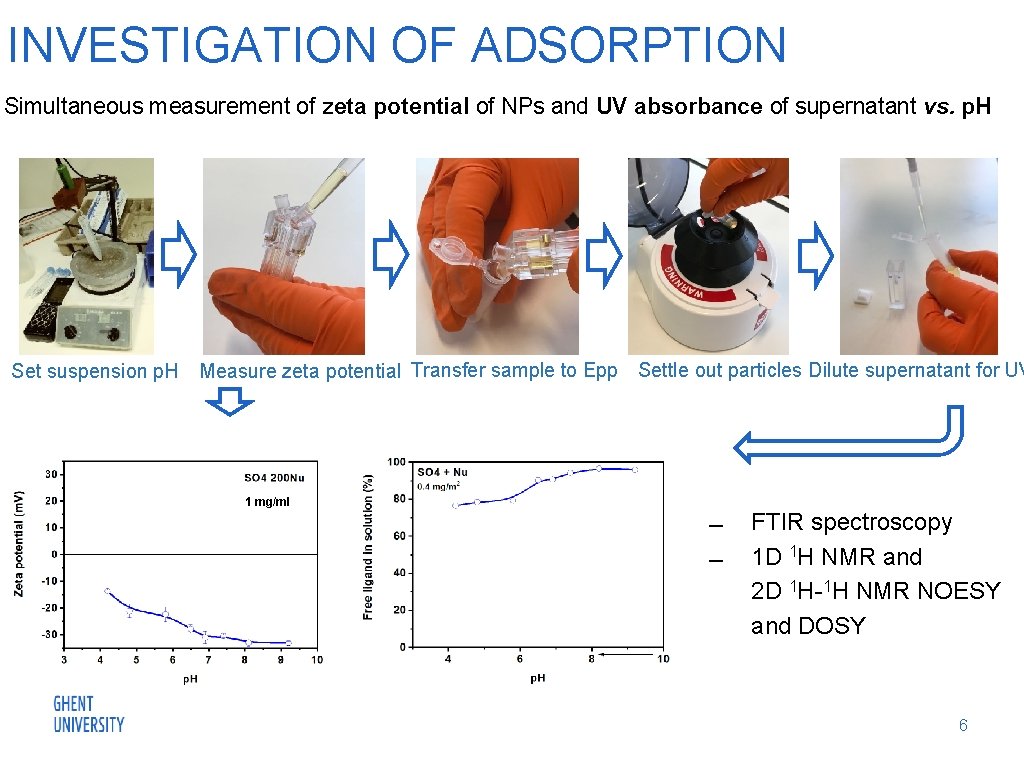
INVESTIGATION OF ADSORPTION Simultaneous measurement of zeta potential of NPs and UV absorbance of supernatant vs. p. H Set suspension p. H Measure zeta potential Transfer sample to Epp Settle out particles Dilute supernatant for UV 1 mg/ml FTIR spectroscopy 1 D 1 H NMR and 2 D 1 H-1 H NMR NOESY and DOSY 6

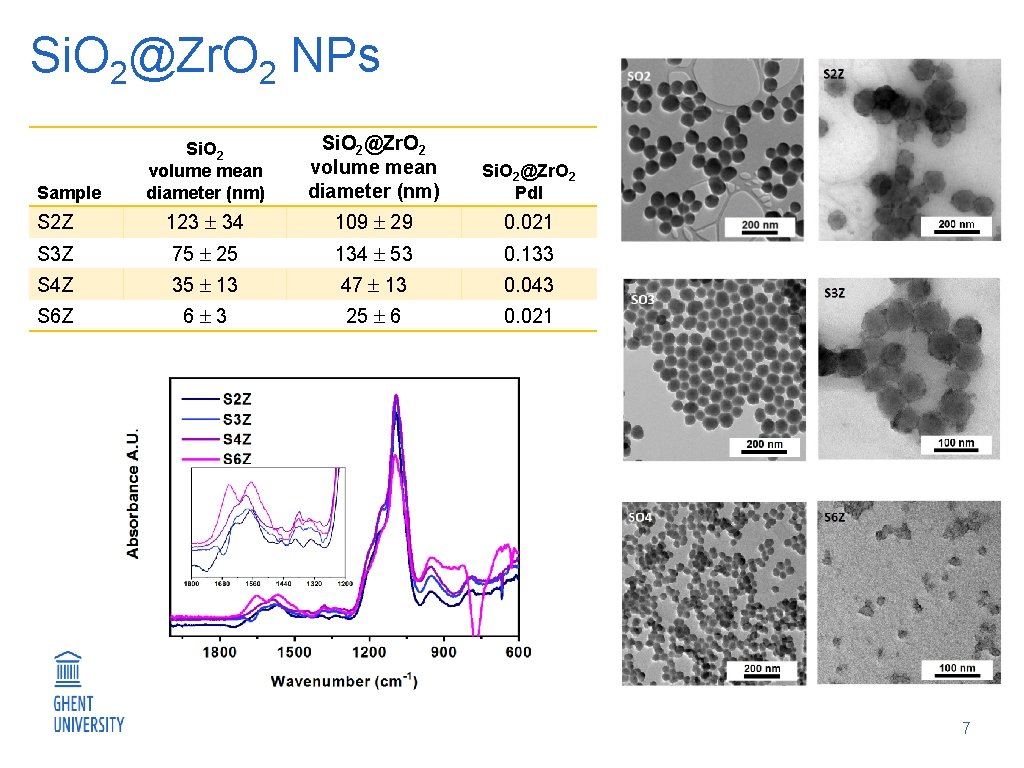
Si. O 2@Zr. O 2 NPs Si. O 2 volume mean diameter (nm) Si. O 2@Zr. O 2 Pd. I S 2 Z 123 34 109 29 0. 021 S 3 Z 75 25 134 53 0. 133 S 4 Z 35 13 47 13 0. 043 S 6 Z 6 3 25 6 0. 021 Sample 7

RESULTS OF SURFACE MODIFICATION 8

ADSORPTION OF NUCLEOSIDE MPs when added to ethanolic sol Nu mixture: whitening + stabilizing effect FTIR: base-pair formation, Phosphate-group binding ZETA POTENTIAL OF DIALYZED SAMPLES d. AMP d. CMP d. GMP TMP NU NATIVE S 2 Z -0. 2 ± 0. 0 m. V -6. 3 ± 0. 6 m. V -53. 0 ± 1. 0 m. V -30. 3 ± 0. 5 m. V -22. 2 ± 0. 3 m. V 11. 9 ± 0. 2 9

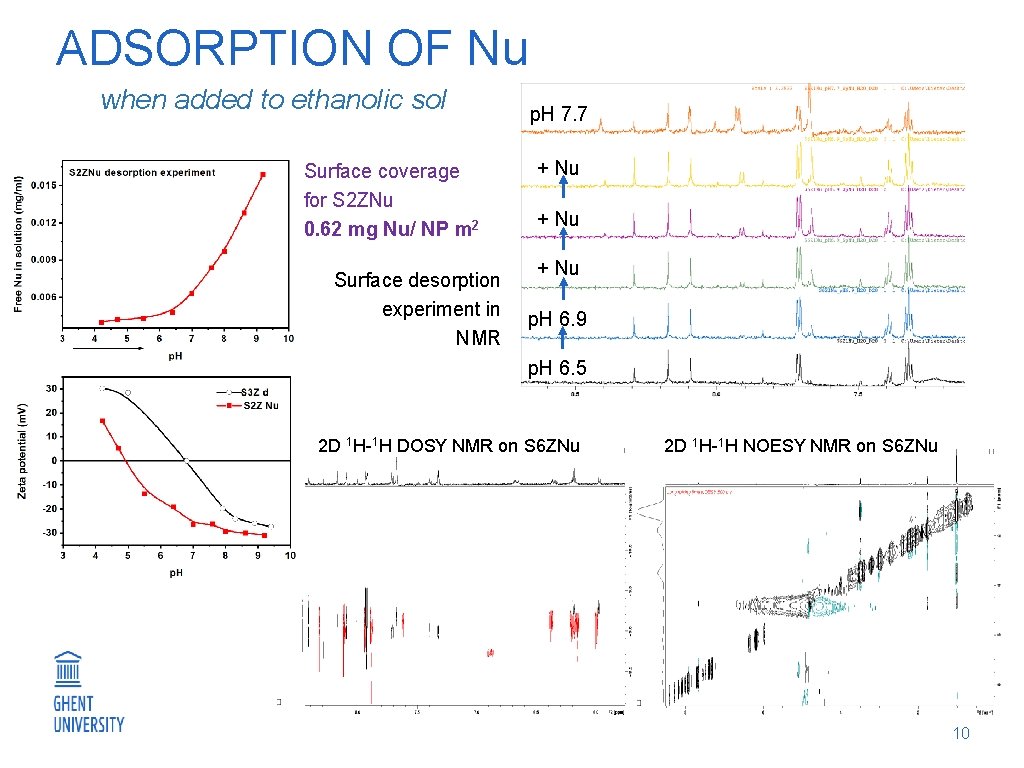
ADSORPTION OF Nu when added to ethanolic sol Surface coverage for S 2 ZNu 0. 62 mg Nu/ NP m 2 Surface desorption experiment in NMR p. H 7. 7 + Nu p. H 6. 9 p. H 6. 5 2 D 1 H-1 H DOSY NMR on S 6 ZNu 2 D 1 H-1 H NOESY NMR on S 6 ZNu 10

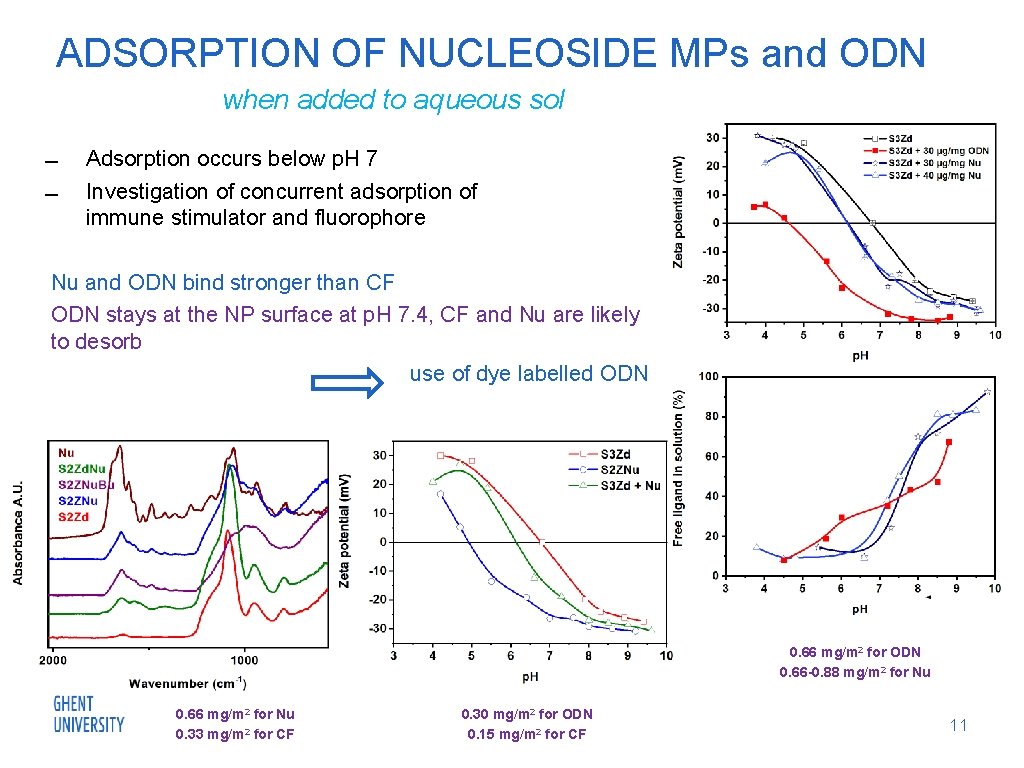
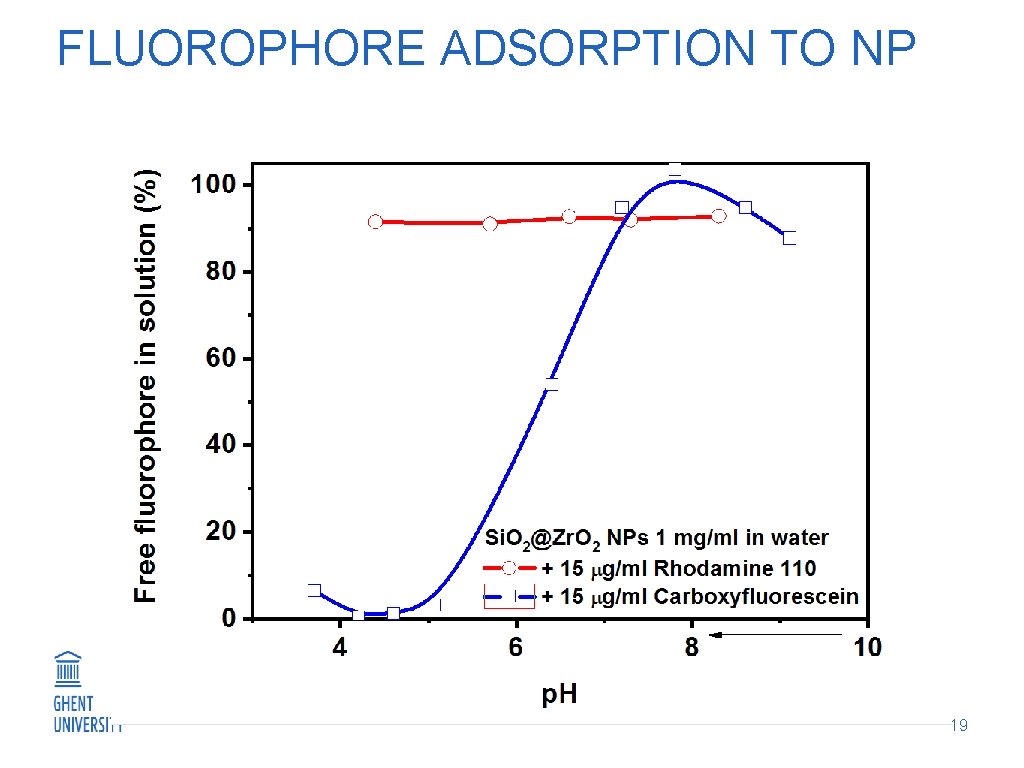
ADSORPTION OF NUCLEOSIDE MPs and ODN when added to aqueous sol Adsorption occurs below p. H 7 Investigation of concurrent adsorption of immune stimulator and fluorophore Nu and ODN bind stronger than CF ODN stays at the NP surface at p. H 7. 4, CF and Nu are likely to desorb use of dye labelled ODN 0. 66 mg/m 2 for ODN 0. 66 -0. 88 mg/m 2 for Nu 0. 66 mg/m 2 for Nu 0. 33 mg/m 2 for CF 0. 30 mg/m 2 for ODN 0. 15 mg/m 2 for CF 11

ADSORPTION OF CF and ODN when added to aqueous sol Detail of 1 D 1 H NMR spectra ODN S 4 Zd + ODN S 6 Z CF + ODN, washed, +ODN S 6 Z CF CF pushed off the surface by ODN in NMR investigation 12

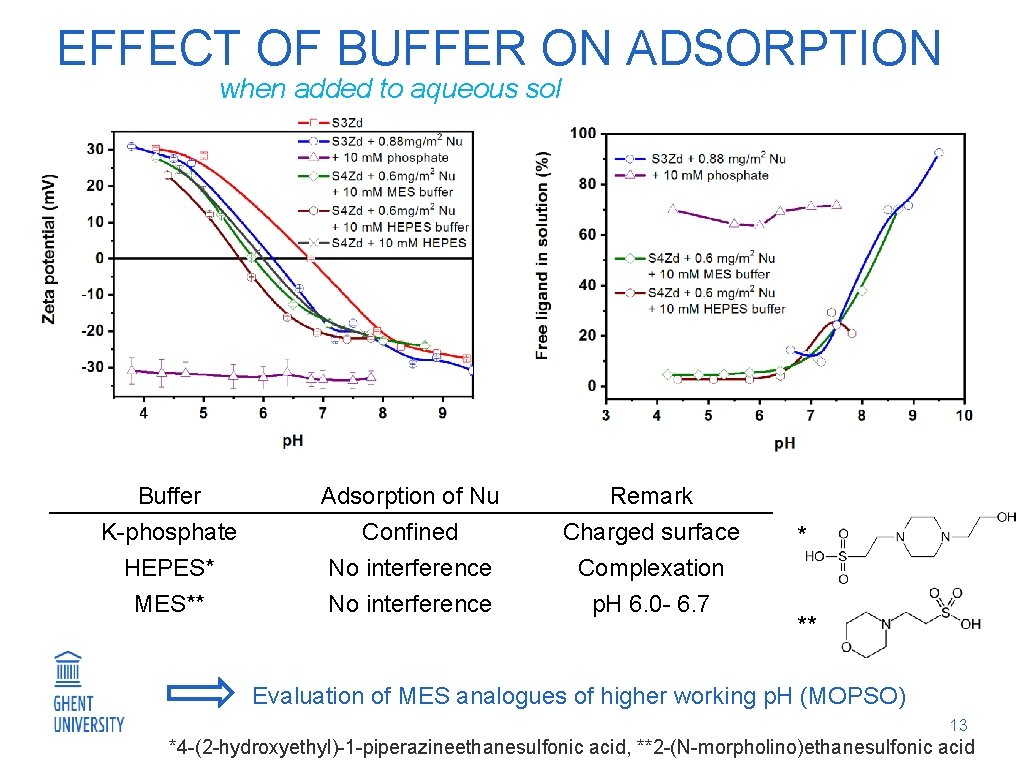
EFFECT OF BUFFER ON ADSORPTION when added to aqueous sol Buffer Adsorption of Nu Remark K-phosphate HEPES* MES** Confined No interference Charged surface Complexation p. H 6. 0 - 6. 7 * ** Evaluation of MES analogues of higher working p. H (MOPSO) 13 *4 -(2 -hydroxyethyl)-1 -piperazineethanesulfonic acid, **2 -(N-morpholino)ethanesulfonic acid

ADSORPTION OF NUCLEOSIDE MPs and ODN when added to aqueous sol H 2 O S 6 Zd + Nu in basic water 0. 1 mg Nu / m 2 NP H 2 O S 4 Zd + ODN in basic water 0. 6 mg ODN / m 2 NP S 6 Zd + Nu in basic water Broad signals in 1 D 1 H NMR The sharp lines give negative NOEs at 500 ms mixing time 14

SUMMARY Surface modification of Si. O 2@Zr. O 2 NPs in ethanol Ligands precipitate onto the NP surface: very little “real” adsorption Higher cargo in water Pure adsorption of ligands Lower amount of Nu adsorbed Addition of ligands in DMSO Use FITC-labeled ODN Find non-interfering buffer 15

ACKNOWLEDGEMENT Promotor: José C. Martins Mentor: Krisztina Fehér Synthetic and analytical laboratory facilities to Prof. Isabel Van Driessche (SCRIPTS group), Prof. Peter Dubruel (PCN group), Prof. Stefaan de Smedt (Ge. RN group) TEM analysis to Katrien De Keukeleere and Evert Dhaene This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 703374.

Livia Naszályi Nagy Marie Curie postdoctoral researcher DEPARTMENT OF ORGANIC Ghent University AND MACROMOLECULAR CHEMISTRY @ugent Ghent University E Livia. Naszalyi. Nagy@ugent. be T +32 9 264 44 88 M +32 487 126 129 www. ugent. be Thank you for your attention!

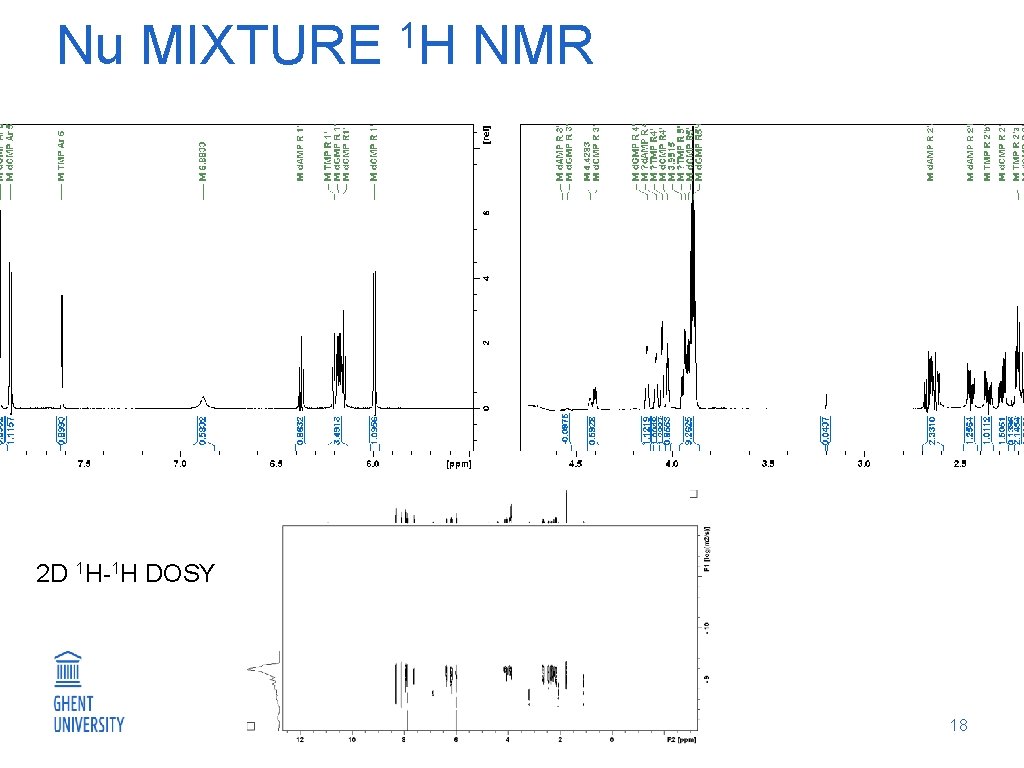
1 Nu MIXTURE H NMR 2 D 1 H-1 H DOSY 18

FLUOROPHORE ADSORPTION TO NP 19

20

Si. O 2@Zr. O 2 – HEPES INTERACTION Appearance of new UV bance peak at 238 nm related to e – HEPES interaction 21

Si. O 2@Zr. O 2 – HEPES INTERACTION Z Nu + 10 m. M HEPES Ga 3+ + HEPES Z Nu + 10 m. M HEPES A. F. Martins et al. Contrast Media Mol. Imaging 22

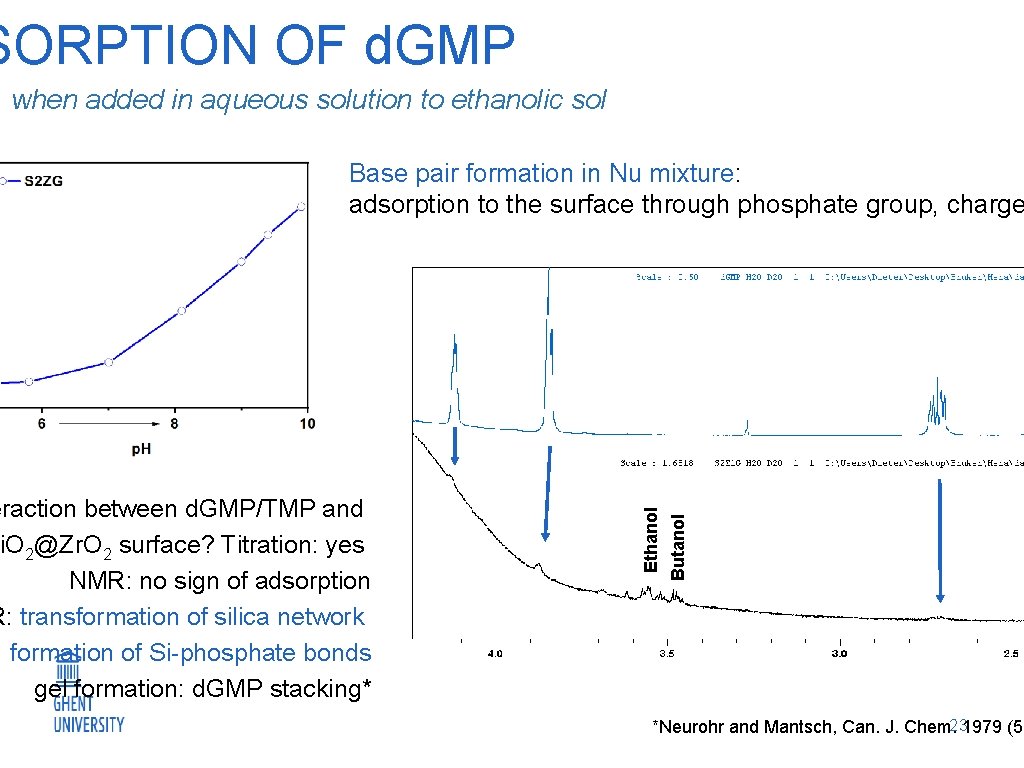
SORPTION OF d. GMP when added in aqueous solution to ethanolic sol Butanol eraction between d. GMP/TMP and i. O 2@Zr. O 2 surface? Titration: yes NMR: no sign of adsorption R: transformation of silica network formation of Si-phosphate bonds gel formation: d. GMP stacking* Ethanol Base pair formation in Nu mixture: adsorption to the surface through phosphate group, charge 23 *Neurohr and Mantsch, Can. J. Chem. 1979 (57

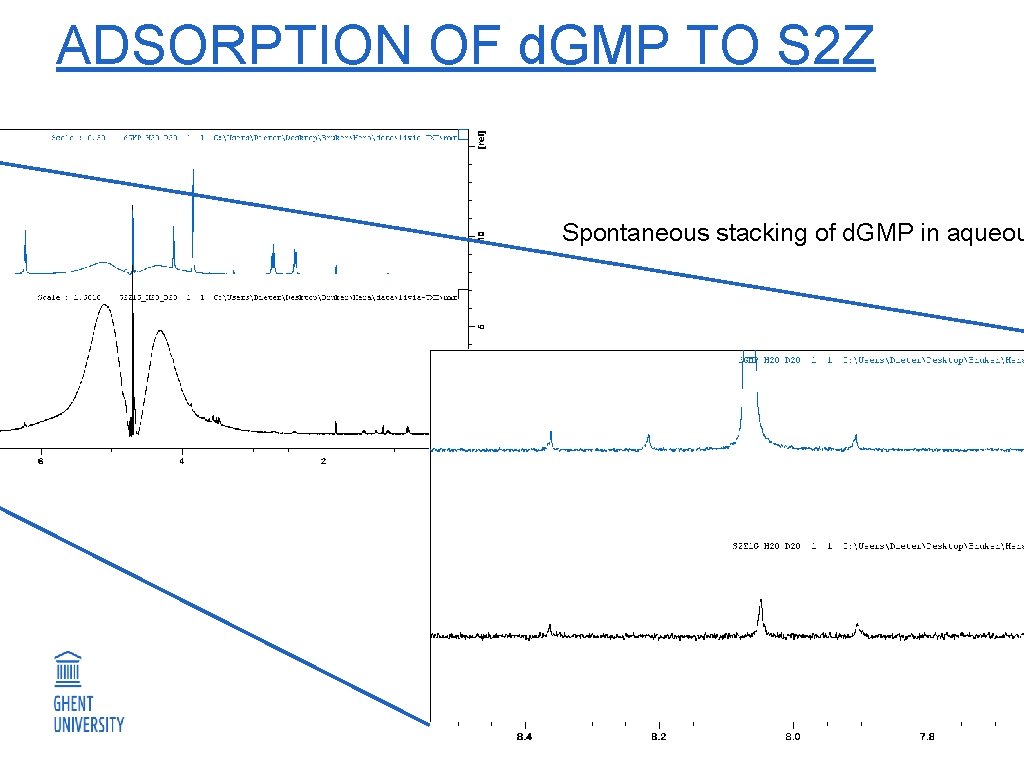
ADSORPTION OF d. GMP TO S 2 Z Spontaneous stacking of d. GMP in aqueou 24

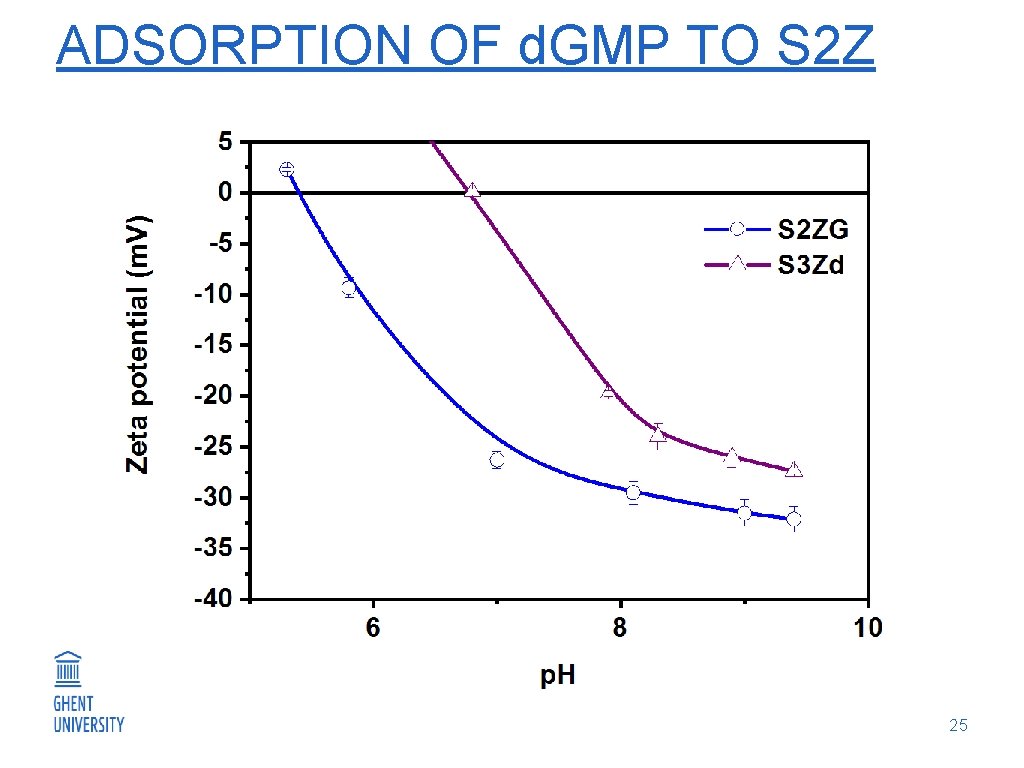
ADSORPTION OF d. GMP TO S 2 Z 25

EFFECT OF HEPES ON ODN ADSORPTION 26
 Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Difference between fats and oil
Difference between fats and oil Lewis dot structure ch4
Lewis dot structure ch4 Enols and enolates organic chemistry
Enols and enolates organic chemistry Numbering carbon chains
Numbering carbon chains Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Alkene family
Alkene family Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s David klein organic chemistry
David klein organic chemistry Is alkane an organic compound
Is alkane an organic compound Ario practice problems
Ario practice problems List of functional groups in order of priority
List of functional groups in order of priority Organic chemistry lab report format
Organic chemistry lab report format Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Importance of organic compounds
Importance of organic compounds Organic chemistry wade
Organic chemistry wade Pent hex hept oct
Pent hex hept oct Cracking organic chemistry
Cracking organic chemistry What is chemistry
What is chemistry Organic chemistry myanmar
Organic chemistry myanmar