Department of Mechanical Engineering ME 322 Mechanical Engineering

- Slides: 16

Department of Mechanical Engineering ME 322 – Mechanical Engineering Thermodynamics Lecture 32 Ideal Gas Mixtures II

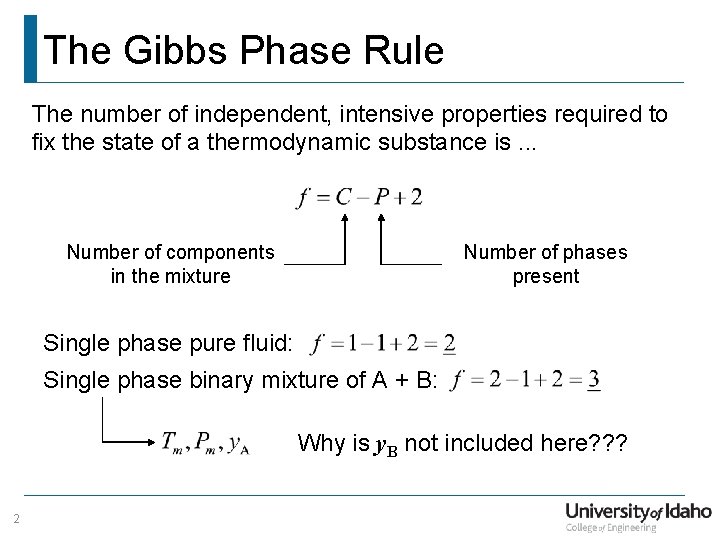
The Gibbs Phase Rule The number of independent, intensive properties required to fix the state of a thermodynamic substance is. . . Number of components in the mixture Number of phases present Single phase pure fluid: Single phase binary mixture of A + B: Why is y. B not included here? ? ? 2

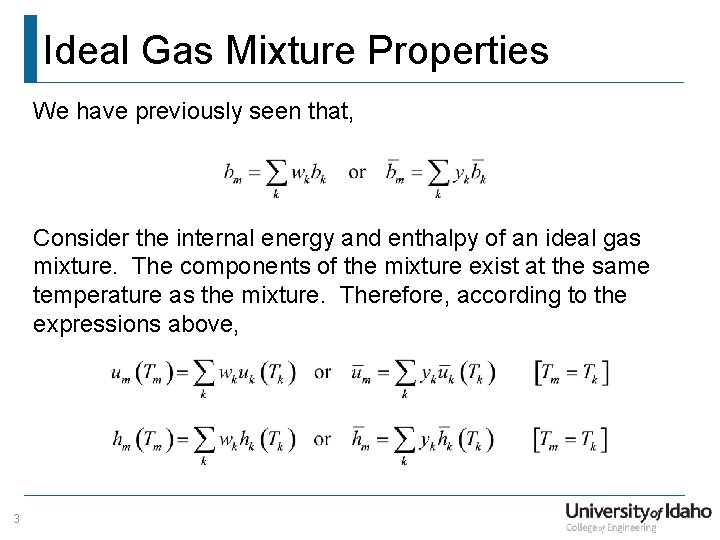
Ideal Gas Mixture Properties We have previously seen that, Consider the internal energy and enthalpy of an ideal gas mixture. The components of the mixture exist at the same temperature as the mixture. Therefore, according to the expressions above, 3

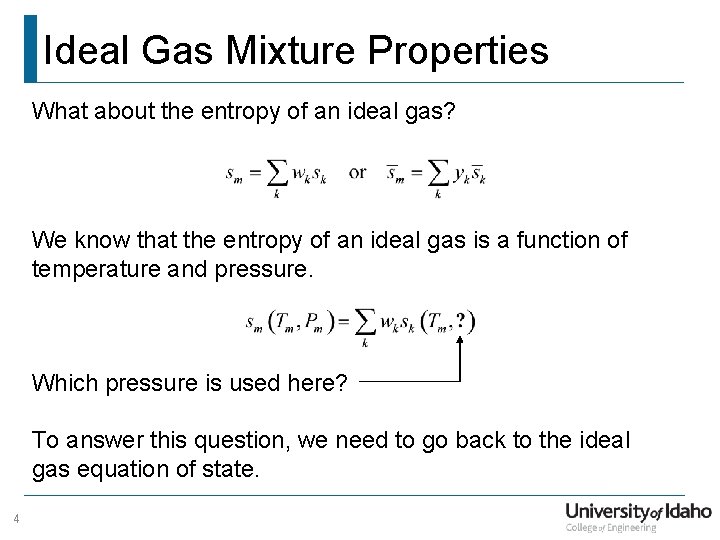
Ideal Gas Mixture Properties What about the entropy of an ideal gas? We know that the entropy of an ideal gas is a function of temperature and pressure. Which pressure is used here? To answer this question, we need to go back to the ideal gas equation of state. 4

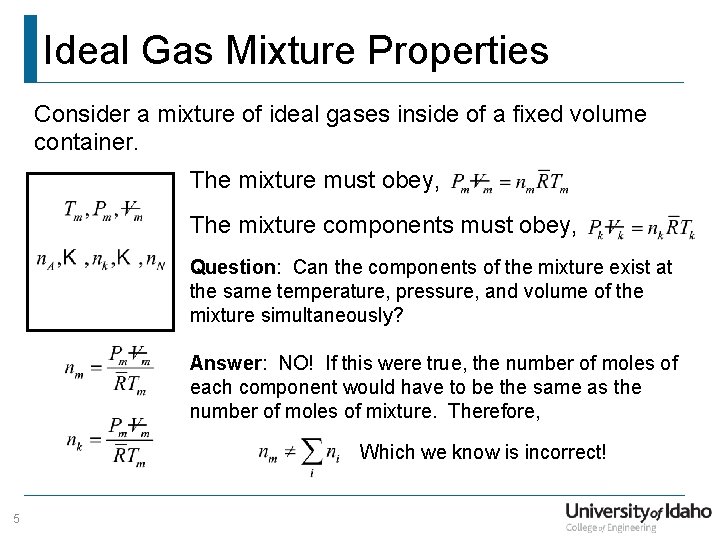
Ideal Gas Mixture Properties Consider a mixture of ideal gases inside of a fixed volume container. The mixture must obey, The mixture components must obey, Question: Can the components of the mixture exist at the same temperature, pressure, and volume of the mixture simultaneously? Answer: NO! If this were true, the number of moles of each component would have to be the same as the number of moles of mixture. Therefore, Which we know is incorrect! 5

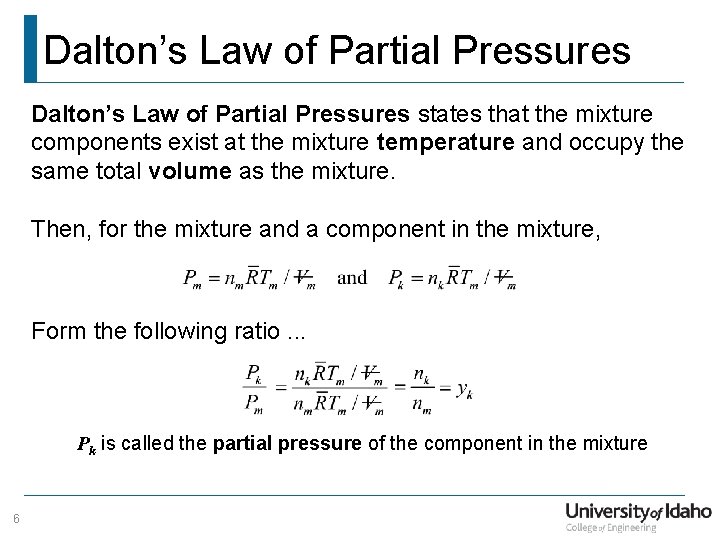
Dalton’s Law of Partial Pressures states that the mixture components exist at the mixture temperature and occupy the same total volume as the mixture. Then, for the mixture and a component in the mixture, Form the following ratio. . . Pk is called the partial pressure of the component in the mixture 6

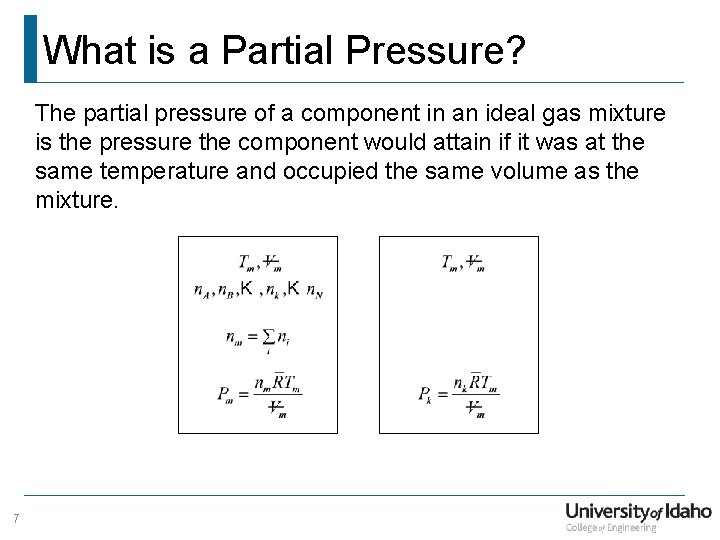
What is a Partial Pressure? The partial pressure of a component in an ideal gas mixture is the pressure the component would attain if it was at the same temperature and occupied the same volume as the mixture. 7

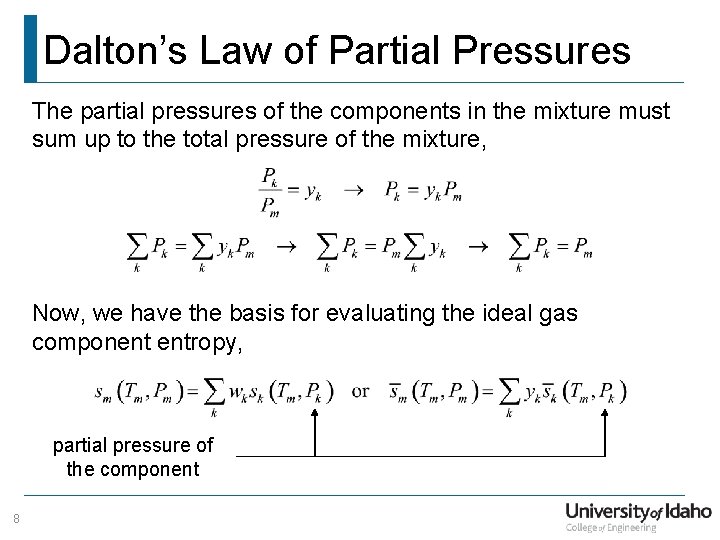
Dalton’s Law of Partial Pressures The partial pressures of the components in the mixture must sum up to the total pressure of the mixture, Now, we have the basis for evaluating the ideal gas component entropy, partial pressure of the component 8

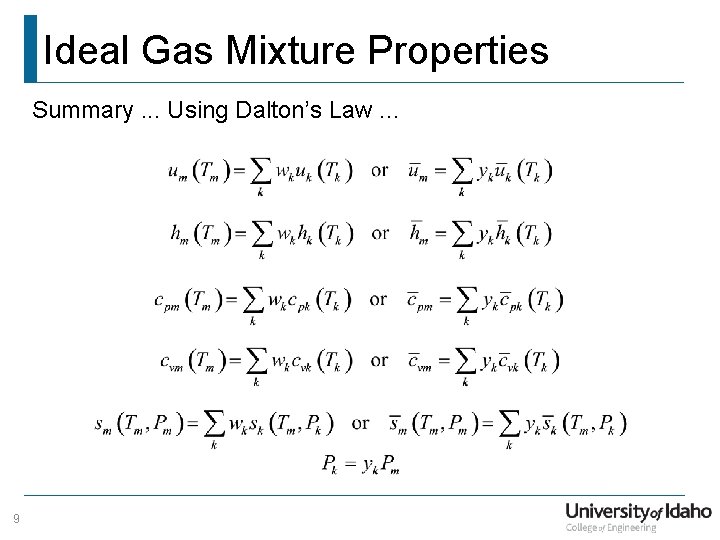
Ideal Gas Mixture Properties Summary. . . Using Dalton’s Law. . . 9

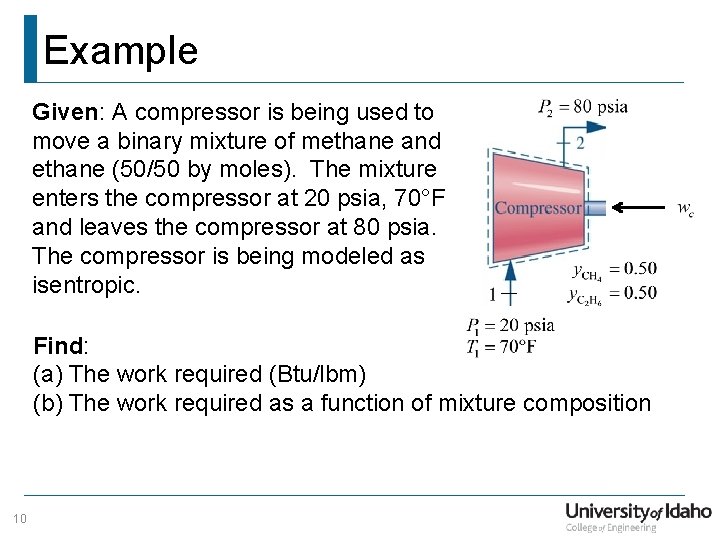
Example Given: A compressor is being used to move a binary mixture of methane and ethane (50/50 by moles). The mixture enters the compressor at 20 psia, 70°F and leaves the compressor at 80 psia. The compressor is being modeled as isentropic. Find: (a) The work required (Btu/lbm) (b) The work required as a function of mixture composition 10

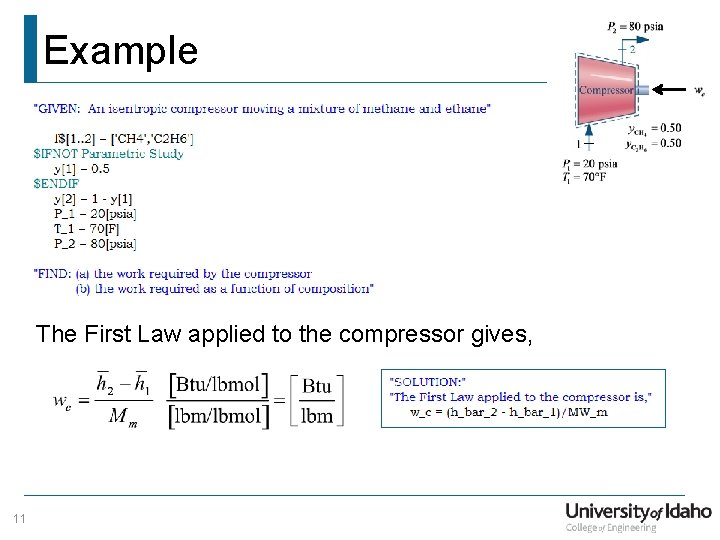
Example The First Law applied to the compressor gives, 11

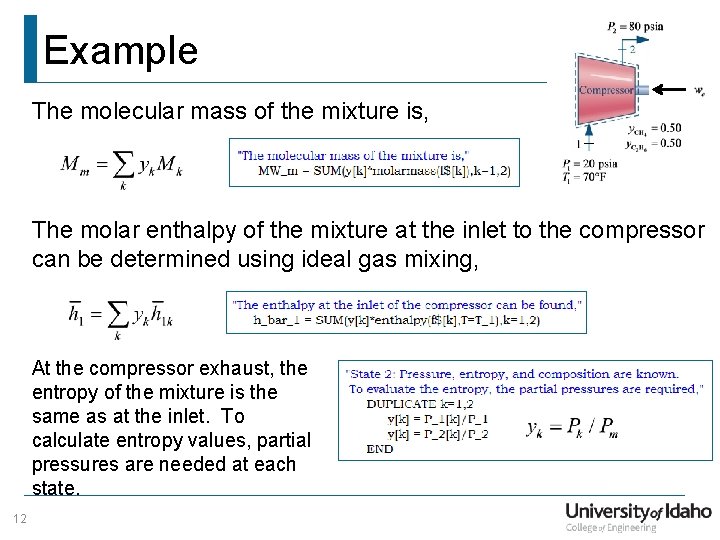
Example The molecular mass of the mixture is, The molar enthalpy of the mixture at the inlet to the compressor can be determined using ideal gas mixing, At the compressor exhaust, the entropy of the mixture is the same as at the inlet. To calculate entropy values, partial pressures are needed at each state. 12

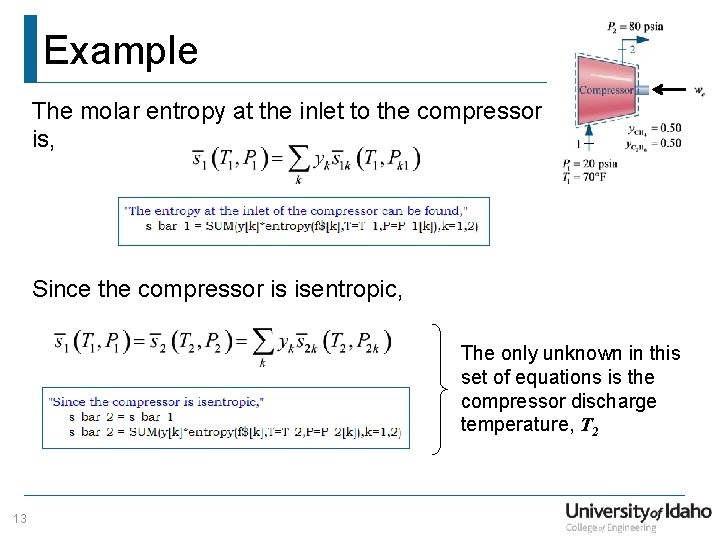
Example The molar entropy at the inlet to the compressor is, Since the compressor is isentropic, The only unknown in this set of equations is the compressor discharge temperature, T 2 13

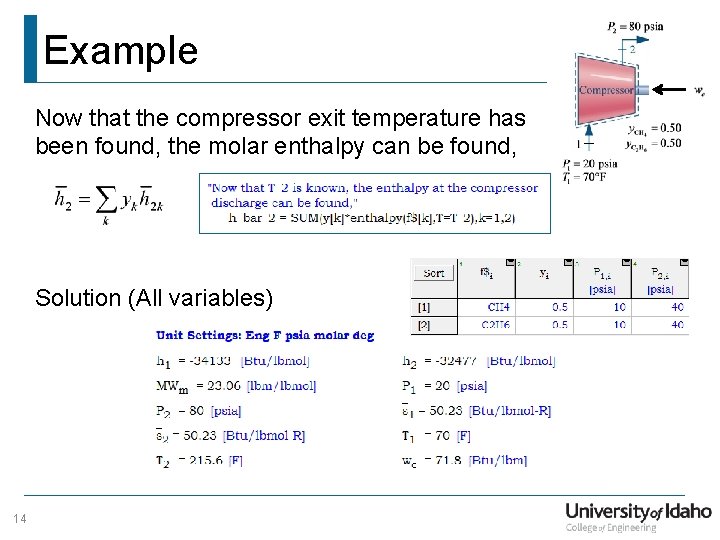
Example Now that the compressor exit temperature has been found, the molar enthalpy can be found, Solution (All variables) 14

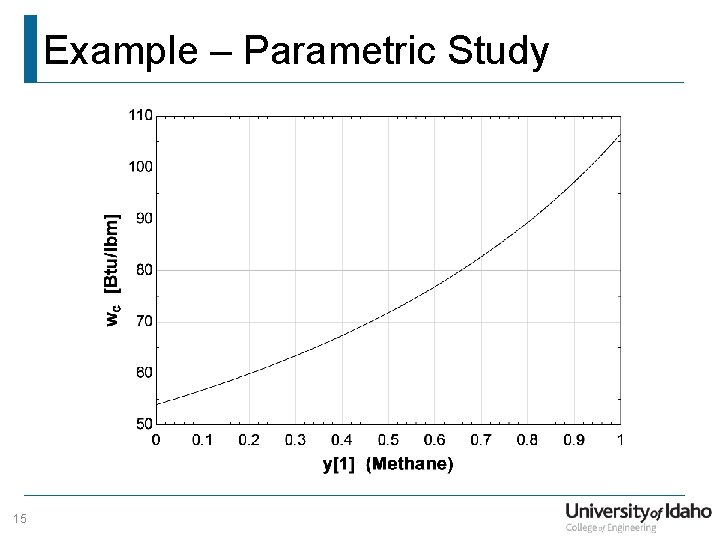
Example – Parametric Study 15

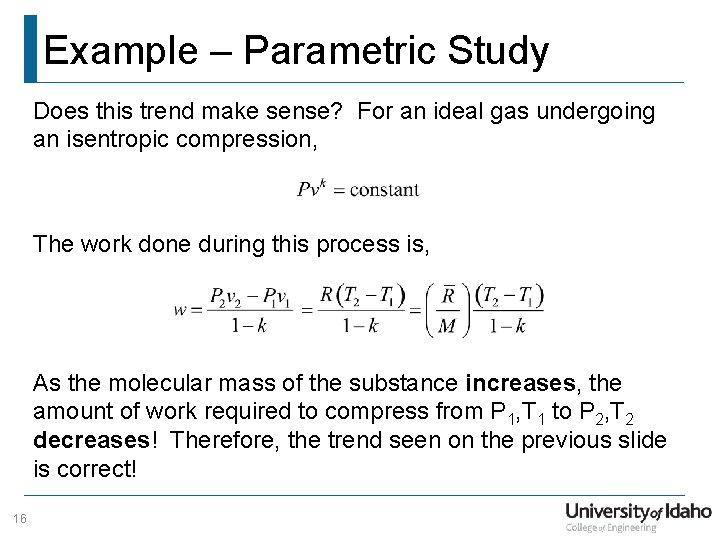
Example – Parametric Study Does this trend make sense? For an ideal gas undergoing an isentropic compression, The work done during this process is, As the molecular mass of the substance increases, the amount of work required to compress from P 1, T 1 to P 2, T 2 decreases! Therefore, the trend seen on the previous slide is correct! 16