Department Of Materials Science Engineering Thermal Treatment of





- Slides: 17

Department Of Materials Science & Engineering Thermal Treatment of Magnox Sludge Intermediate Level Nuclear Waste through Vitrification Sean T. Barlow BSc (Hons) AMInst. P Martin C. Stennett 1, Russell J. Hand 1, Sean P. Morgan 2 & Neil C. Hyatt 1 1. Department of Materials Science & Engineering, The University of Sheffield, Sheffield S 1 3 JD, UK 2. Sellafield Ltd. , Hinton House, Risley, Warrington WA 3 6 GR, UK Scientific Basis for Nuclear Waste Management Symposium 2017, October 29 – November 3 | Sydney, Australia

Department Of Materials Science & Engineering 1956 – Calderhall Worlds first civil nuclear power station

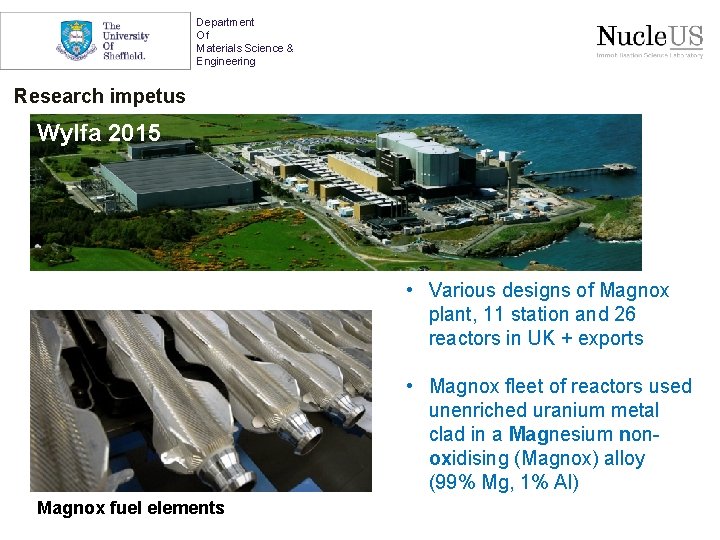
Department Of Materials Science & Engineering Research impetus Wylfa 2015 • Various designs of Magnox plant, 11 station and 26 reactors in UK + exports • Magnox fleet of reactors used unenriched uranium metal clad in a Magnesium nonoxidising (Magnox) alloy (99% Mg, 1% Al) Magnox fuel elements

Department Of Materials Science & Engineering Background First Generation Magnox Storage Pond (FGMSP)

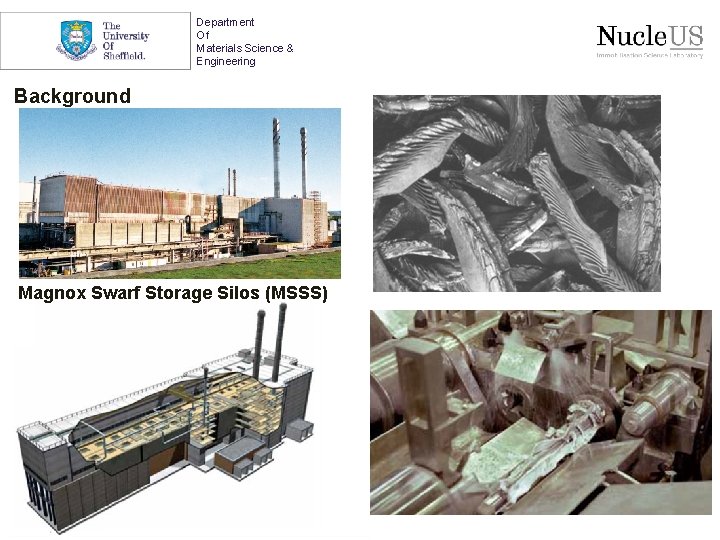
Department Of Materials Science & Engineering Background Magnox Swarf Storage Silos (MSSS)

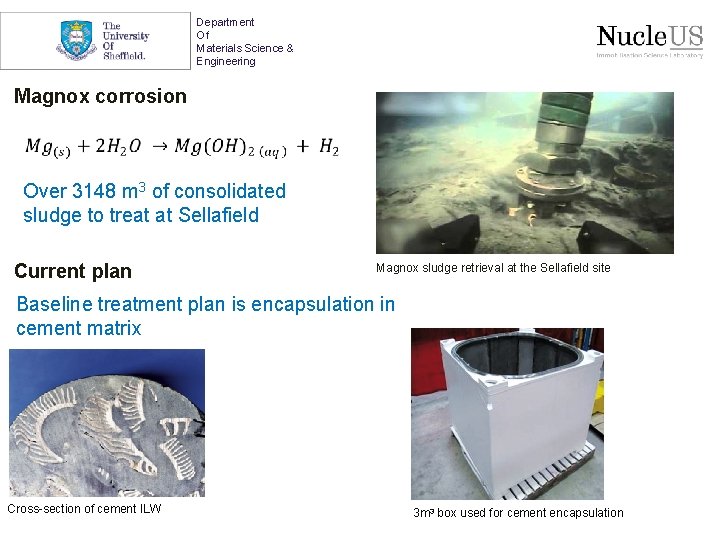
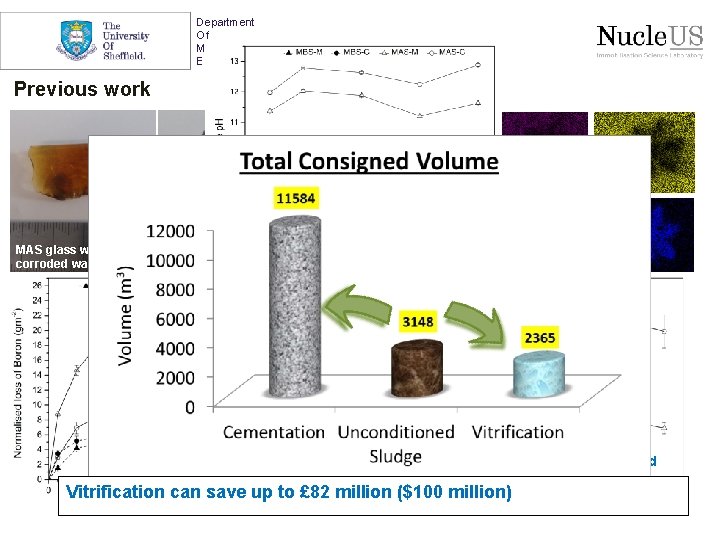
Department Of Materials Science & Engineering Magnox corrosion Over 3148 m 3 of consolidated sludge to treat at Sellafield Current plan Magnox sludge retrieval at the Sellafield site Baseline treatment plan is encapsulation in cement matrix Cross-section of cement ILW 3 m 3 box used for cement encapsulation

Department Of Materials Science & Engineering Current plan Cementation has two key drawbacks: Ø Difficulty predicting long-term durability Ø Significant increase in waste volume Alternative wasteform needed to improve safety case & save on cost of geological disposal

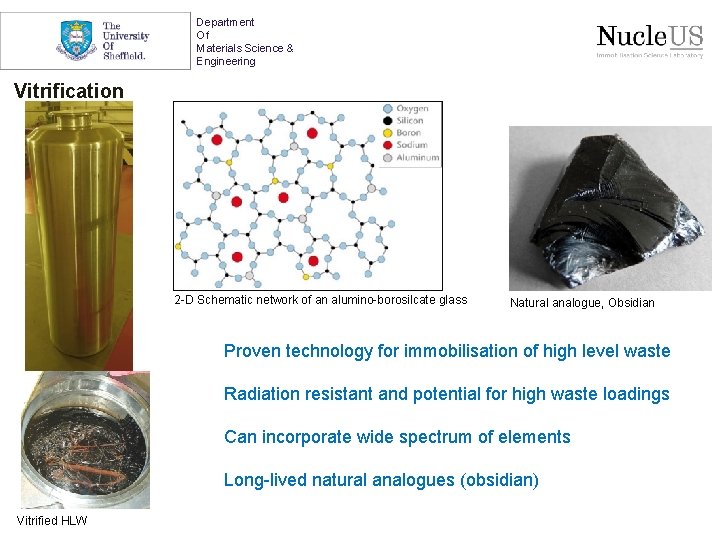
Department Of Materials Science & Engineering Vitrification 2 -D Schematic network of an alumino-borosilcate glass Natural analogue, Obsidian Proven technology for immobilisation of high level waste Radiation resistant and potential for high waste loadings Can incorporate wide spectrum of elements Long-lived natural analogues (obsidian) Vitrified HLW

Department Of Materials Science & Engineering Previous work MAS glass with corroded waste MAS glass with metallic waste MBS glass with corroded waste MBS glass with metallic waste RLB: (1. 40 ± 8. 3) x 10 -2 g/m 2/d MBS-M O Mg Si U RLU: (3. 45 ± 0. 01) x 10 -5 g/m 2/d Between 33 -37 wt% waste Vitrification can saveloadings up to £ 82 million ($100 million)

Department Of Materials Science & Engineering Surrogate compatibility v Use of other elements to simulate uranium v Looked into neodymium and mischmetal (lanthanide alloy) Ø Replaced U in MBS and MAS samples on a molar basis Ø Same melting conditions § 1250 °C for 3 hours (MBS) § 1500 °C for 5 hours (MAS) Nd glass under incandescent light Nd glass under fluorescent light

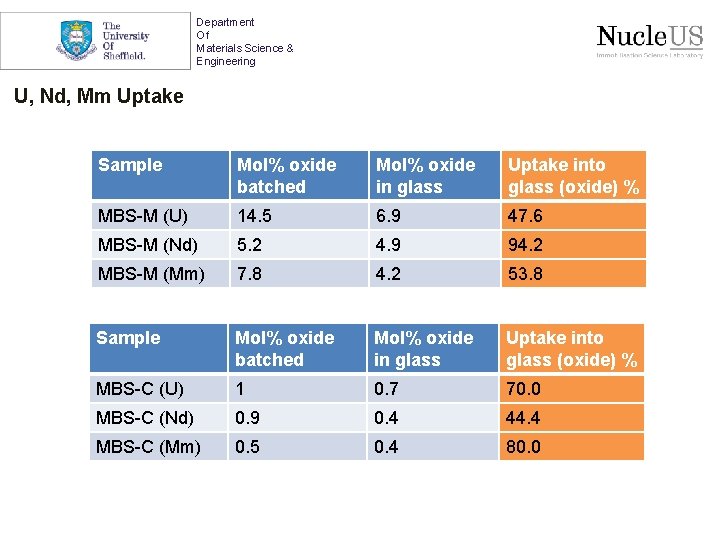
Department Of Materials Science & Engineering U, Nd, Mm Uptake Sample Mol% oxide batched Mol% oxide in glass Uptake into glass (oxide) % MBS-M (U) 14. 5 6. 9 47. 6 MBS-M (Nd) 5. 2 4. 9 94. 2 MBS-M (Mm) 7. 8 4. 2 53. 8 Sample Mol% oxide batched Mol% oxide in glass Uptake into glass (oxide) % MBS-C (U) 1 0. 7 70. 0 MBS-C (Nd) 0. 9 0. 4 44. 4 MBS-C (Mm) 0. 5 0. 4 80. 0

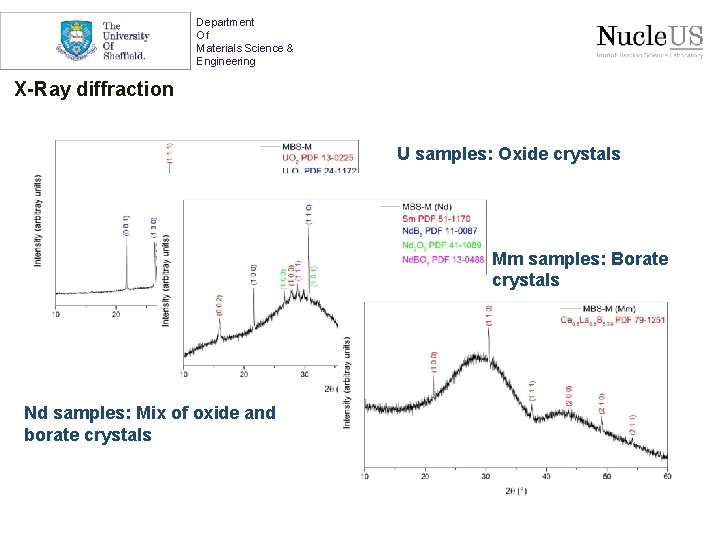
Department Of Materials Science & Engineering X-Ray diffraction U samples: Oxide crystals Mm samples: Borate crystals Nd samples: Mix of oxide and borate crystals

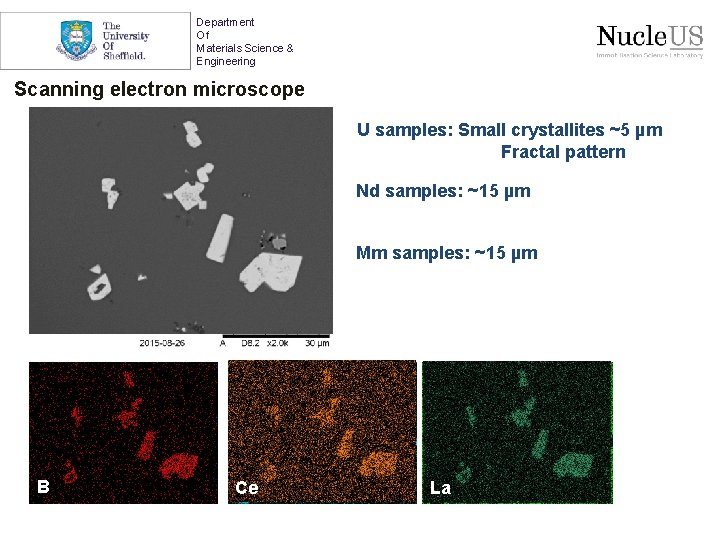
Department Of Materials Science & Engineering Scanning electron microscope U samples: Small crystallites ~5 µm Fractal pattern Nd samples: ~15 µm Mm samples: ~15 µm B Nd Ce Si La

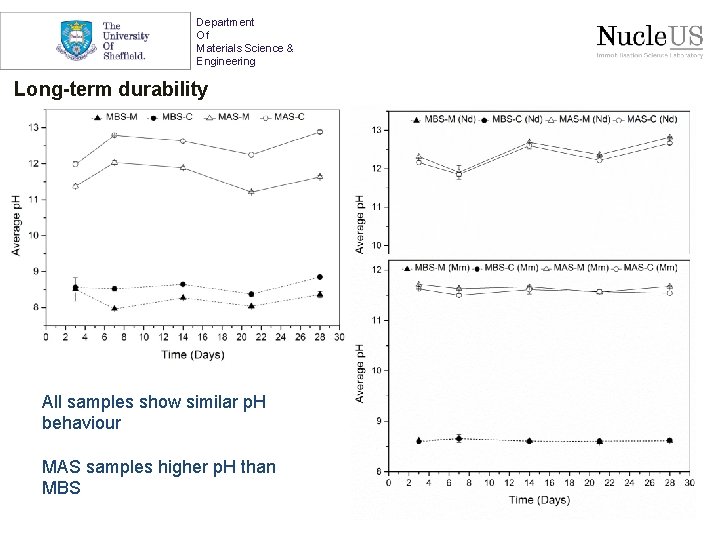
Department Of Materials Science & Engineering Long-term durability All samples show similar p. H behaviour MAS samples higher p. H than MBS

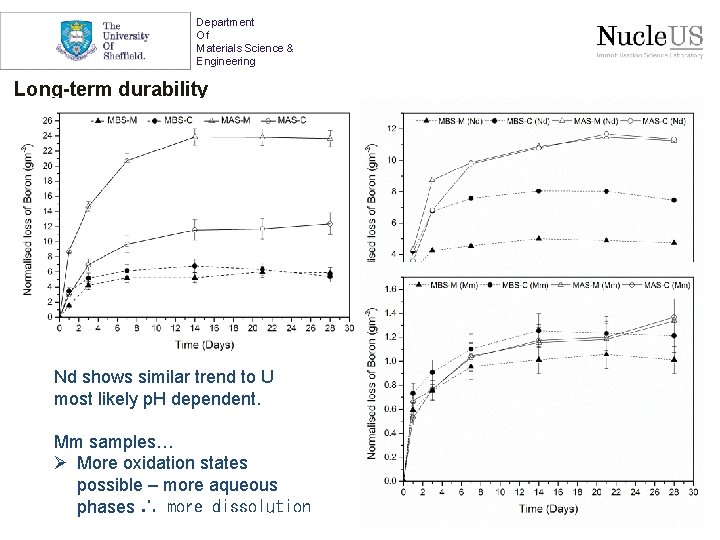
Department Of Materials Science & Engineering Long-term durability Nd shows similar trend to U most likely p. H dependent. Mm samples… Ø More oxidation states possible – more aqueous phases ∴ more dissolution

Department Of Materials Science & Engineering Conclusions & Further Work • A single glass formulation (MBS) can be used to treat both extremes of the waste expected in Sellafield’s Magnox sludge – previous study. • Mischmetal comparable to Uranium for uptake into glass structure • Neodymium and Mischmetal for borate phases – larger crystal size than uranium • Neodymium comparable for dissolution studies, Mm larger amount of OS possible Ø Determine the oxidation state of Nd and Mm samples Ø Raman spectroscopy – structure of the glass

Department Of Materials Science & Engineering Special thanks to: Prof Neil C. Hyatt Prof Russell J. Hand Dr Martin C. Stennett Dr Claire L. Corkhill Dr Sean P. Morgan Mr Adam J. Fisher Mr Daniel J. Bailey & the ISL team Thank you for listening! The authors would like to thank the EPSRC (Grant EP/G 037140/1) for funding this research which was performed in part at the MIDAS Facility, University of Sheffield, established with support from the Department of Energy and Climate Change.