Department of Health and Human Services IVD Validation

















- Slides: 24

Department of Health and Human Services IVD Validation and Regulation in Rx/Dx Combinations FDA/Industry Statistics Workshop Classifiers in Combination Rx/Dx Submissions Robert L. Becker, Jr, MD, Ph. D U. S. Food and Drug Administration Center for Devices and Radiological Health Office of In Vitro Diagnostic Device Evaluation and Safety

Department of Health and Human Services Setting • Coordinated, interdependent development and use of diagnostic devices and therapeutics is both needed and happening now. - Preclinical drug development Focusing drug trials Shaping drug indications (test, then treat) Measuring drug effect (treat, then test…) 2

Department of Health and Human Services Practical Constraints • Diagnostic devices measuring biomarkers have technical characteristics and limitations, demonstrated from experience, that inform their use. • Biomarker measurement is a messy affair, with challenges that affect the ease of Rx/Dx application. • Study designs for biomarker/IVD validation, and clearance or approval, present trade-offs. • Regulatory approach must accommodate all of the above. 3

Department of Health and Human Services What’s coming… • Serological tumor markers • Histological tumor markers • Recent Applications - CD 117 - Her 2/neu - EGFR 4

Department of Health and Human Services Serological Markers • CA 19 -9, CA 125, CA 15 -3, CEA, AFP - Monitoring (510(k)) • PSA - Monitoring, total (510(k)) - Diagnosis, free and total (PMA) 5

Department of Health and Human Services Practical Challenges in Validation • Analytical - Sensitivity Specificity Accuracy Precision Cut-off Linearity • Clinical - Cut-off Sensitivity Specificity Dose response • Clinical Utility - Population - Individual 6

Department of Health and Human Services 7

Department of Health and Human Services Tumor Associated Antigen Immunological Test System “Measurement of tumor-associated antigen levels may aid in the monitoring of patients for disease progression or response to therapy or for the detection of recurrent or residual disease. Tumorassociated antigen immunoassay systems intended for use in screening for the early detection or diagnosis of cancer in either the general population or in a high risk population, or in disease staging, are not included. ” 8

Department of Health and Human Services TAA Uses and Impacts • Diagnosis - Screening - Confirmatory initial diagnosis - Residual or recurrent disease • Monitoring - Change in tumor burden over time • Prognosis - Likely outcome (e. g. natural history), given a set of features • Prediction - Marker-dependent change in outcome, given a new or changed therapy 9

Department of Health and Human Services Histological Tumor-Associated Markers • Reviewed (history) - Immunohistochemistry - Gene amplification (FISH) • Not yet reviewed (future) - Gene expression (m. RNA) - Gene imbalance (CGH) - Somatic mutations 10

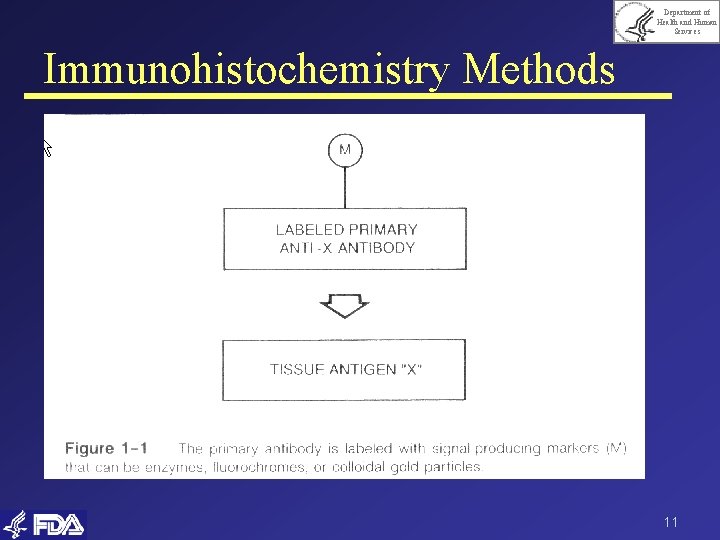
Department of Health and Human Services Immunohistochemistry Methods 11

Department of Health and Human Services A Few IHC Complications • Non-linear amplification, signal “development” • Antigen recovery, variation • Antibody specificity, affinity, avidity • Readout variates (distribution, intensity, prevalence) • As a result, analytical (and hence clinical) sensitivity and specificity are highly dependent on technique. 12

Department of Health and Human Services FISH vs IHC Techniques • FISH probes and ligands usually better defined • FISH uses less layering or amplification • FISH cytologic features more discrete; possibly easier readout • Multiple (e. g. two) markers readily accomodated • Technique aside, what is clinical import? Less widely studied, but this is changing. 13

Department of Health and Human Services Immunohistological Markers • Long and wide (TNTC) experience with markers of tumor histogenesis – generally Class 1 exempt • Long but narrower (e. g. down-classified ER/PR) experience with markers for prognosis or prediction • Recent experience with a few markers intended to help guide chemotherapy selection. 14

Department of Health and Human Services 15

Department of Health and Human Services Immunohistochemical Applications “Class I IHC’s provide the pathologist with adjunctive diagnostic information that may be incorporated into the pathologist’s report, but that is not ordinarily reported to the clinician as an independent finding. ” “Class II IHC’s are intended for the detection and/or measurement of certain target analytes by immunological techniques in order to provide prognostic and predictive data that are not directly confirmed by routine histopathologic internal and external congtrol specimens. These IHC’s provide the pathologist with diagnostic information that is ordinarily reported as independent diagnostic information to the ordering clinician, and the claims associated with these data are widely accepted and supported by valid scientific evidence. ” “[Class III IHC’s] are IHC’s that do not meet the criteria for class II, or are IHC’s that meet those criteria but raise new issues of safety and effectiveness. ” 16

Department of Health and Human Services Three Recent PMA IHC Applications • CD 117 (c-kit) for imatinib (Gleevec) treatment of gastrointestinal stromal tumor • Her 2/neu for trastuzumab (Herceptin) treatment of metastatic breast cancer • EGFR for cetuximab (Erbitux) or panitumumab (Vectibix) treatment of metastatic colorectal cancer • None of these applications included a prospective trial of the device for its ability to predict drug response. 17

Department of Health and Human Services Absent a BM+/BM- Drug Trial… • Biomarker predictive value for drug effect is incompletely evaluated at best. - Rely on BM-dependent drug effect within BM+ patients. Risk exclusion of potentially responsive patients. Cannot dissect BM predictive power from prognostic power. How much confidence in the BM IVD cut-off? In the assay to meet it? Post-approval study commitments? Fulfillable? What non-trial evidence suffices to conclude non-response for BMpatients? - Larger problem when BM+ fraction is small. • Some combination of practical benefits to trial execution. - Lower cost? - More power? - Fewer adverse events? 18

Department of Health and Human Services CD 117 and Gleevec for GIST • “…indicated as an aid in the diagnosis of GIST within the context of the patient’s clinical history, tumor morphology, and other diagnostic tests… …may be used after the diagnosis of GIST as an aid in the selection of GIST patients who may qualify for imatinib mesylate (Gleevec) therapy. ” • Any specific staining is a positive result. • Main utility is in helping to identify GIST, not in selecting the drug. 19

Department of Health and Human Services Her-2/neu IHC and Herceptin for Breast Ca • “…indicated as an aid in the assessment of patients for whom HERCEPTIN (Trastuzumab) treatment is being considered…” • Graded staining result (2+ vs 3+ makes a difference) • Technique and read-out variations, in deployed performance, may decrease effectiveness – FISH back-up for IHC 2+ cases. 20

Department of Health and Human Services EGFR and Erbitux for Colorectal CA • “…indicated as an aid in identifying colorectal cancer patients eligible for treatment with ERBITUX…” • No sign of clinical response dependence on IHC signal strength • Post-market suggestions that IHC “negative” patients respond similar to “positives” • Ambiguity as to what is a “negative” result 21

Department of Health and Human Services Some Issues to Address • Analytical validation of IHC tests, the earlier the better, especially wrt definition and performance near cut-off points. • Trial designs such that clinical validity is assessed across the full range of test results (i. e. including “negative” patients). • Retention and access to clinical trial samples so that later tests (either same or different technique) can be properly evaluated. 22

Department of Health and Human Services Why worry? • Multiple modalities for tumor assessment aimed at drug selection are emerging – EGFR for NSCLC as an example. • Other markers further complicate the picture. • Numerous non-comparable, low-power studies. • Risk that biomarkers will be unfairly dismissed, or relied on without justification. 23

Department of Health and Human Services Why hope? • Issues, though complex and controversial, can at least be defined. • Continually improving coordination between stakeholders. • With large stakes, continuing interest seems assured. 24
 Iowa department of health and human services
Iowa department of health and human services Milwaukee county department of health and human services
Milwaukee county department of health and human services Maine department of health and human services
Maine department of health and human services Washington state department of social and health services
Washington state department of social and health services Department of health and senior services missouri
Department of health and senior services missouri Ivd product development process
Ivd product development process Rudolf koch ivd
Rudolf koch ivd Companion diagnostic regulation
Companion diagnostic regulation Ivd 4 pint
Ivd 4 pint Ivd coating wiki
Ivd coating wiki Edma ivd
Edma ivd Seattle human services department
Seattle human services department Illinois mental health collaborative
Illinois mental health collaborative Delaware county human services
Delaware county human services Louisiana department of health and hospitals licensing
Louisiana department of health and hospitals licensing Milwaukee county dhhs
Milwaukee county dhhs Siskiyou county human services
Siskiyou county human services Barnstable county human services
Barnstable county human services Wake county human services community services center
Wake county human services community services center Human validation process
Human validation process Fl dept of agriculture
Fl dept of agriculture Virginia department of agriculture food safety
Virginia department of agriculture food safety Mussd
Mussd Barnstable county department of health and environment
Barnstable county department of health and environment Vcaa hhd
Vcaa hhd