Department of Chemical Engineering Nara National College of


- Slides: 16

Department of Chemical Engineering Nara National College of Technology Electrochemical Stability of Au sub-ML on Pt/QC Electrode Nara National college of technology Takanori KOBAYASHI, Atsuhiro KAWAMURA, Katsumi KATAKURA, Hirohisa YAMADA

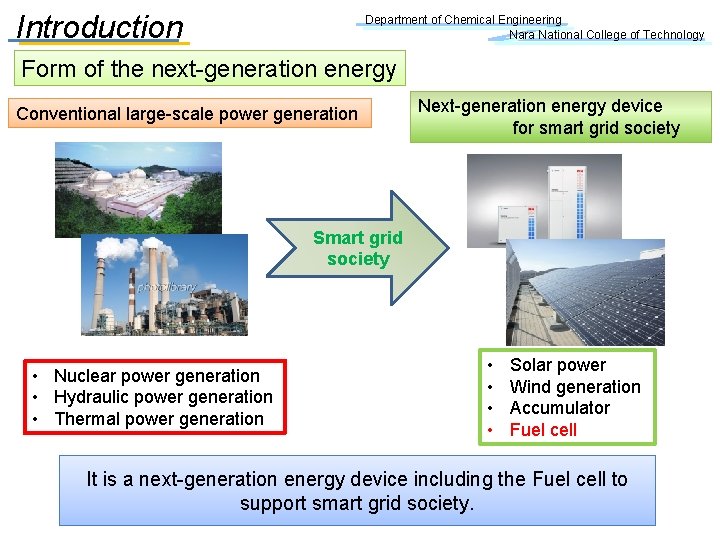
Introduction Department of Chemical Engineering Nara National College of Technology Form of the next-generation energy Conventional large-scale power generation Next-generation energy device for smart grid society Smart grid society • Nuclear power generation • Hydraulic power generation • Thermal power generation • • Solar power Wind generation Accumulator Fuel cell It is a next-generation energy device including the Fuel cell to support smart grid society.

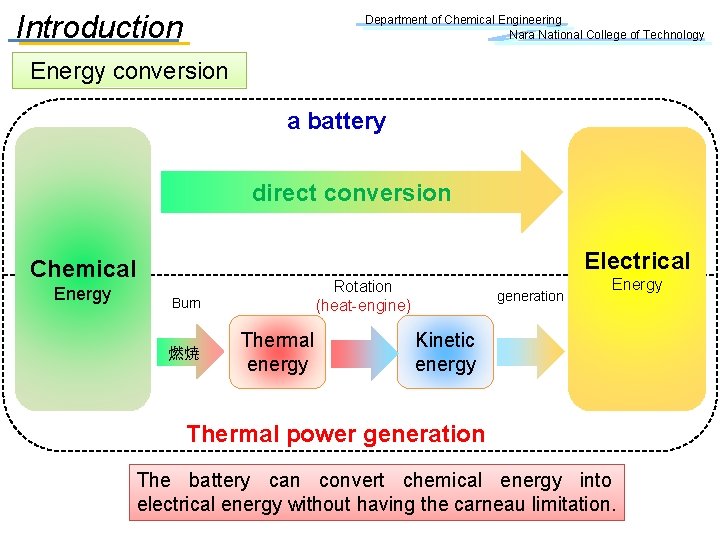
Introduction Department of Chemical Engineering Nara National College of Technology Energy conversion a battery direct conversion Electrical Chemical Energy Burn 燃焼 Rotation (heat-engine) Thermal energy generation Energy Kinetic energy Thermal power generation The battery can convert chemical energy into electrical energy without having the carneau limitation.

Introduction Department of Chemical Engineering Nara National College of Technology Polymer Electrolyte Fuel Cells (PEFCs) O 2(air) Reformed H 2 e- Pt nanoparticle H+ Separetor Electrolyte CP(Carbon Paper) Pt catalyst Fig. 1 Schematic illustration for PEFCs. Carbon black Fig. 2 Schematic illustration for Pt/C catalyst.

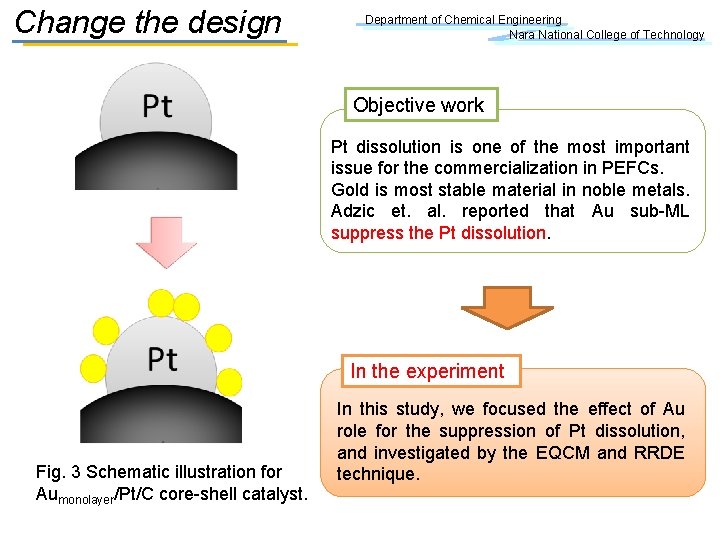
Change the design Department of Chemical Engineering Nara National College of Technology Objective work Pt dissolution is one of the most important issue for the commercialization in PEFCs. Gold is most stable material in noble metals. Adzic et. al. reported that Au sub-ML suppress the Pt dissolution. In the experiment Fig. 3 Schematic illustration for Aumonolayer/Pt/C core-shell catalyst. In this study, we focused the effect of Au role for the suppression of Pt dissolution, and investigated by the EQCM and RRDE technique.

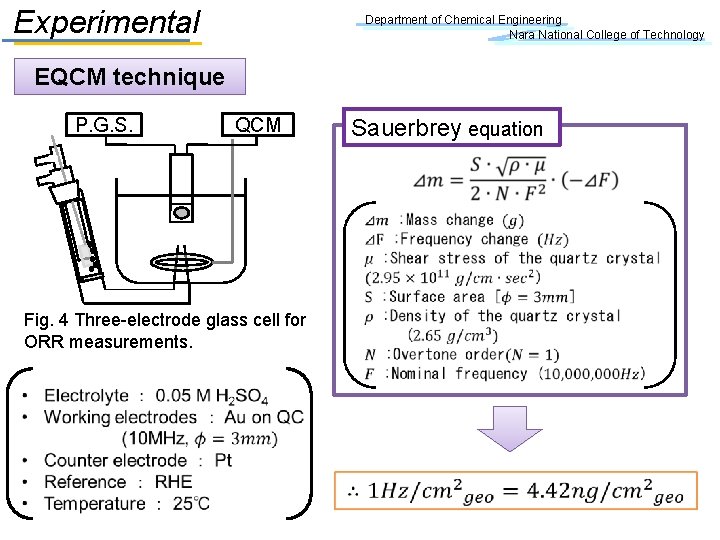
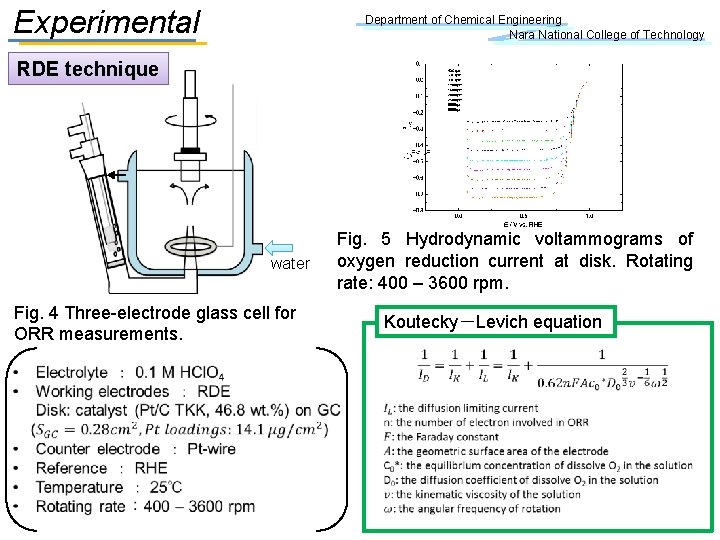
Experimental Department of Chemical Engineering Nara National College of Technology EQCM technique P. G. S. Sauerbrey equation QCM Fig. 4 Three-electrode glass cell for ORR measurements.

Experimental Department of Chemical Engineering Nara National College of Technology RDE technique water Fig. 4 Three-electrode glass cell for ORR measurements. Fig. 5 Hydrodynamic voltammograms of oxygen reduction current at disk. Rotating rate: 400 – 3600 rpm. Koutecky-Levich equation

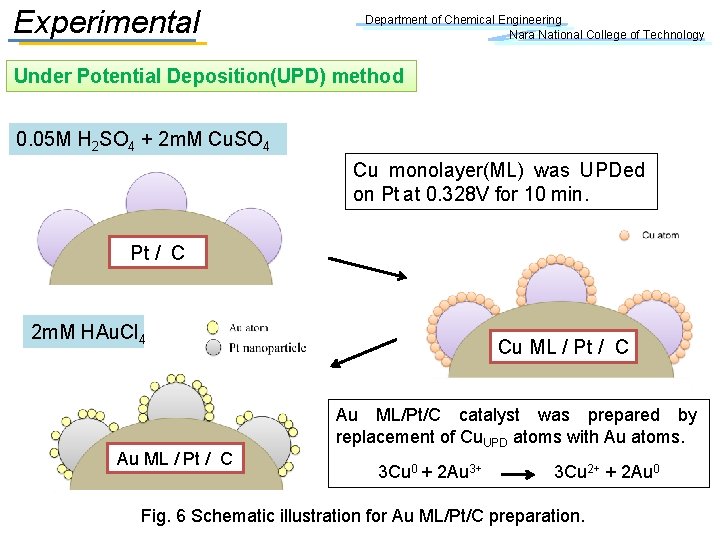
Experimental Department of Chemical Engineering Nara National College of Technology Under Potential Deposition(UPD) method 0. 05 M H 2 SO 4 + 2 m. M Cu. SO 4 Cu monolayer(ML) was UPDed on Pt at 0. 328 V for 10 min. Pt / C 2 m. M HAu. Cl 4 Au ML / Pt / C Cu ML / Pt / C Au ML/Pt/C catalyst was prepared by replacement of Cu. UPD atoms with Au atoms. 3 Cu 0 + 2 Au 3+ 3 Cu 2+ + 2 Au 0 Fig. 6 Schematic illustration for Au ML/Pt/C preparation.

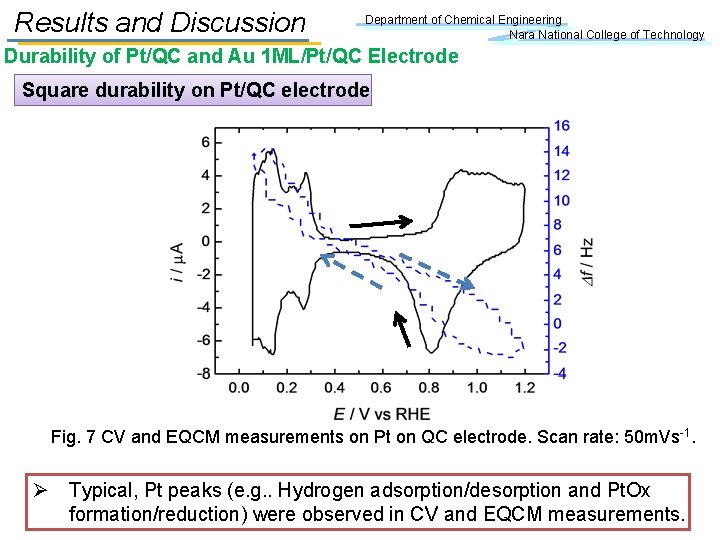
Results and Discussion Department of Chemical Engineering Nara National College of Technology Durability of Pt/QC and Au 1 ML/Pt/QC Electrode Square durability on Pt/QC electrode Fig. 7 CV and EQCM measurements on Pt on QC electrode. Scan rate: 50 m. Vs-1. Ø Typical, Pt peaks (e. g. . Hydrogen adsorption/desorption and Pt. Ox formation/reduction) were observed in CV and EQCM measurements.

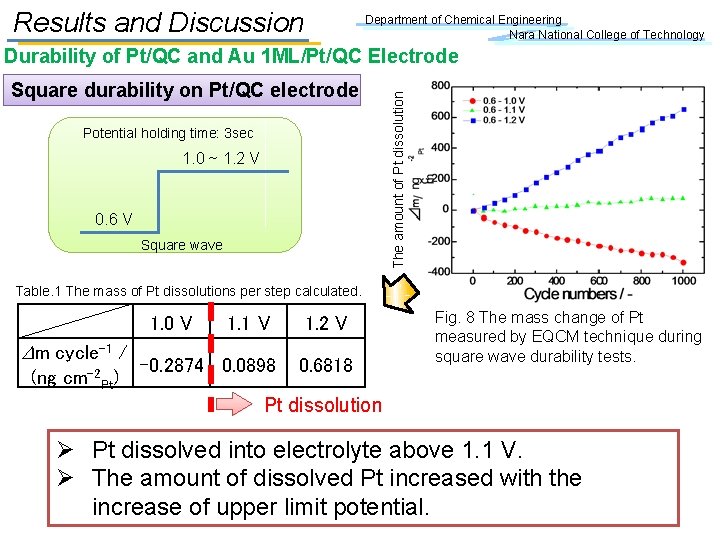
Results and Discussion Department of Chemical Engineering Nara National College of Technology Square durability on Pt/QC electrode Potential holding time: 3 sec 1. 0 ~ 1. 2 V 0. 6 V Square wave The amount of Pt dissolution Durability of Pt/QC and Au 1 ML/Pt/QC Electrode Table. 1 The mass of Pt dissolutions per step calculated. 1. 0 V 1. 1 V 1. 2 V Dm cycle-1 / -0. 2874 0. 0898 0. 6818 (ng cm-2 Pt) Pt dissolution Fig. 8 The mass change of Pt measured by EQCM technique during square wave durability tests. Ø Pt dissolved into electrolyte above 1. 1 V. Ø The amount of dissolved Pt increased with the increase of upper limit potential.

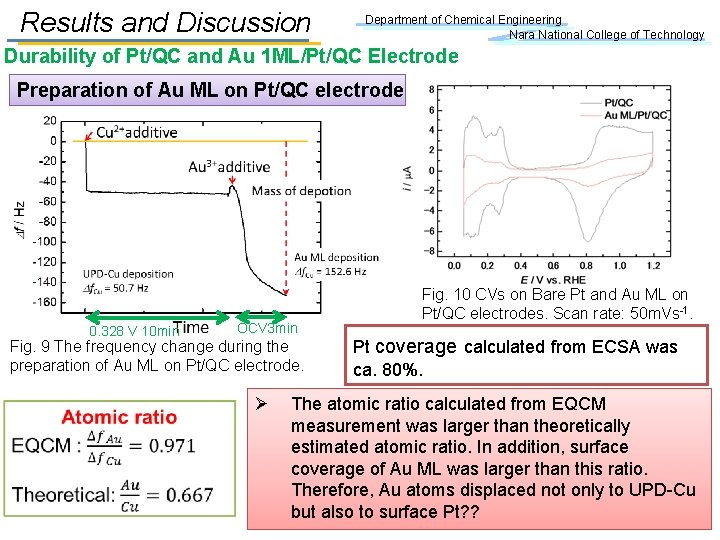
Results and Discussion Department of Chemical Engineering Nara National College of Technology Durability of Pt/QC and Au 1 ML/Pt/QC Electrode Preparation of Au ML on Pt/QC electrode 0. 328 V 10 min OCV 3 min Fig. 9 The frequency change during the preparation of Au ML on Pt/QC electrode. Ø Fig. 10 CVs on Bare Pt and Au ML on Pt/QC electrodes. Scan rate: 50 m. Vs-1. Pt coverage calculated from ECSA was ca. 80%. The atomic ratio calculated from EQCM measurement was larger than theoretically estimated atomic ratio. In addition, surface coverage of Au ML was larger than this ratio. Therefore, Au atoms displaced not only to UPD-Cu but also to surface Pt? ?

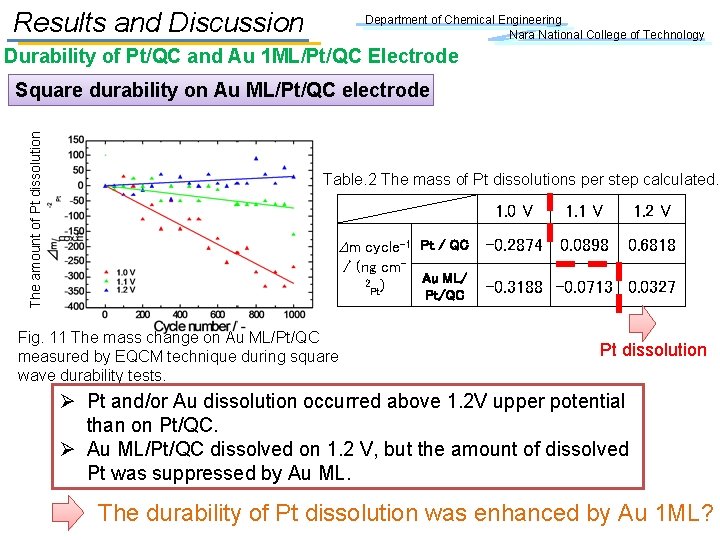
Results and Discussion Department of Chemical Engineering Nara National College of Technology Durability of Pt/QC and Au 1 ML/Pt/QC Electrode The amount of Pt dissolution Square durability on Au ML/Pt/QC electrode Table. 2 The mass of Pt dissolutions per step calculated. 1. 0 V Dm cycle-1 Pt / QC -0. 2874 / (ng cm. Au ML/ 2 ) Pt Pt/QC Fig. 11 The mass change on Au ML/Pt/QC measured by EQCM technique during square wave durability tests. 1. 1 V 1. 2 V 0. 0898 0. 6818 -0. 3188 -0. 0713 0. 0327 Pt dissolution Ø Pt and/or Au dissolution occurred above 1. 2 V upper potential than on Pt/QC. Ø Au ML/Pt/QC dissolved on 1. 2 V, but the amount of dissolved Pt was suppressed by Au ML. The durability of Pt dissolution was enhanced by Au 1 ML?

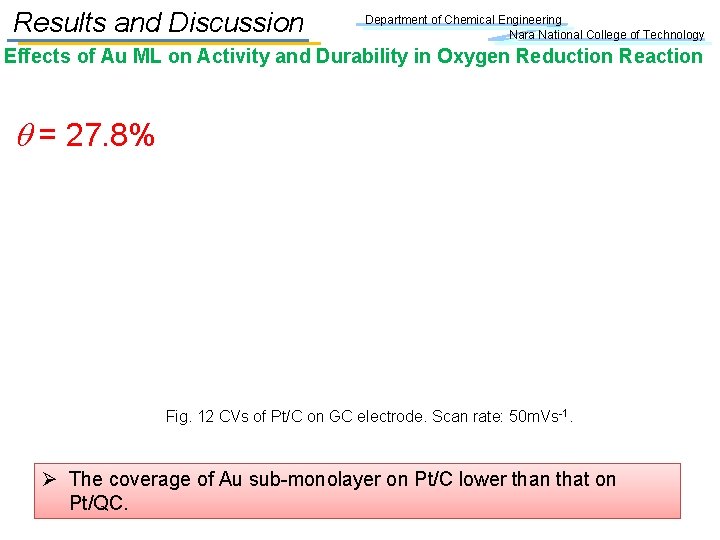
Results and Discussion Department of Chemical Engineering Nara National College of Technology Effects of Au ML on Activity and Durability in Oxygen Reduction Reaction q = 27. 8% Fig. 12 CVs of Pt/C on GC electrode. Scan rate: 50 m. Vs-1. Ø The coverage of Au sub-monolayer on Pt/C lower than that on Pt/QC.

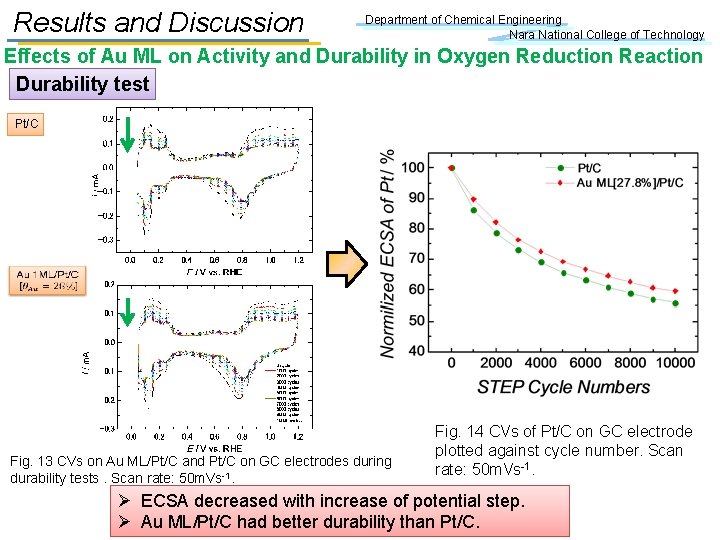
Results and Discussion Department of Chemical Engineering Nara National College of Technology Effects of Au ML on Activity and Durability in Oxygen Reduction Reaction Durability test Pt/C Fig. 13 CVs on Au ML/Pt/C and Pt/C on GC electrodes during durability tests. Scan rate: 50 m. Vs-1. Fig. 14 CVs of Pt/C on GC electrode plotted against cycle number. Scan rate: 50 m. Vs-1. Ø ECSA decreased with increase of potential step. Ø Au ML/Pt/C had better durability than Pt/C.

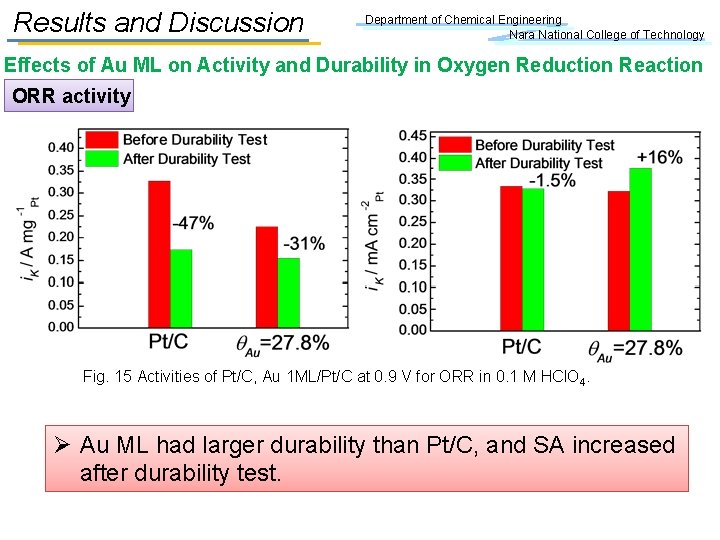
Results and Discussion Department of Chemical Engineering Nara National College of Technology Effects of Au ML on Activity and Durability in Oxygen Reduction Reaction ORR activity Fig. 15 Activities of Pt/C, Au 1 ML/Pt/C at 0. 9 V for ORR in 0. 1 M HCl. O 4. Ø Au ML had larger durability than Pt/C, and SA increased after durability test.

Conclusions Department of Chemical Engineering Nara National College of Technology
 Nara images
Nara images Nara records retention policy
Nara records retention policy Nara women's university
Nara women's university Japan's first capital city
Japan's first capital city Gordon everett nara
Gordon everett nara Arcis login
Arcis login Nara licensing
Nara licensing Nara records management training
Nara records management training Nara takeda
Nara takeda Nara images
Nara images Raju venugopalan
Raju venugopalan How would you describe the new capital city nara
How would you describe the new capital city nara How would you describe the new capital city, nara?
How would you describe the new capital city, nara? Pasadena city college police department
Pasadena city college police department What is a national risk ambulance
What is a national risk ambulance Core standards of a health establishments in south africa
Core standards of a health establishments in south africa Function of national audit department in malaysia
Function of national audit department in malaysia