Dental Standards Testing Methods Types of tests for
























- Slides: 31

Dental Standards & Testing Methods

Types of tests for biomaterials • Lab Tests • Clinical Tests

Clinical Tests – Advantages: • simulates the temperature, humidity, and fluid in the environment • simulates the mechanical forces exerted during mastication

Clinical Tests – Disadvantages: • patients highly variable – need large numbers: cost • specimens often not recoverable • studies take a very long time: cost • results may be skewed toward making the material look good

Clinical Tests – Sources of bias: • patient selection • skilled operators • study environment – University practices may not simulate typical dental practices • desire to please corporate sponsors

Laboratory Testing – advantages: • quicker and consequently cheaper • specimens can be recovered • no danger to patients or animals • testing conditions are repeatable – patient variability is not a factor

Laboratory Testing – disadvantages: • correct mechanical conditions are difficult to know • correct testing environment (temperature, saliva chemistry, p. H, etc. ) is difficult to know

Laboratory Testing – two types of tests: • use tests try to simulate oral conditions (e. g. , cyclic testing, 37 o. C, artificial saliva, etc. ) • abuse tests exaggerate clinical conditions – try to quickly produce results that predict long-term durability of materials

US Standards: 1920 U. S. Army supports Wilmer Souder at the Nat’l Bureau of Standards 1920 -28 Weinstein Fellowships at the NBS

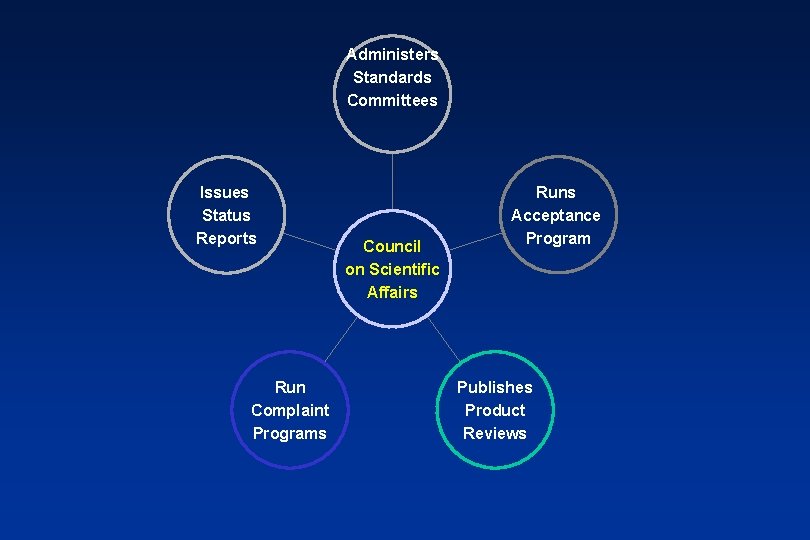
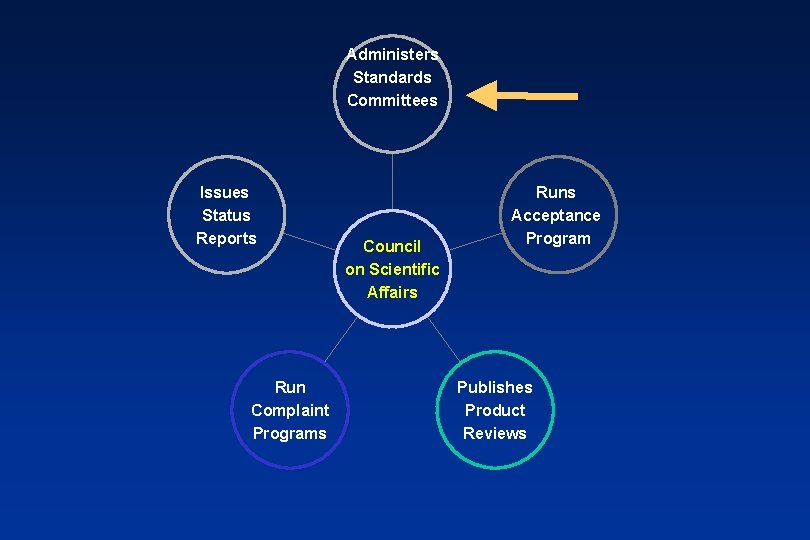
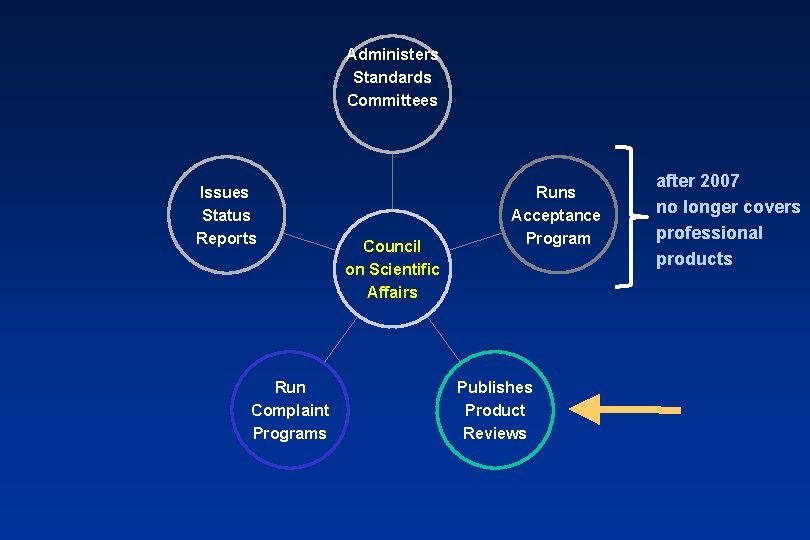
US Standards: 1928 - ADA Fellowships support research at the NBS Over 20 Specifications are developed 1966 - ADA establishes Council on Dental Materials & Devices. More recently this council was merged with others to become the ADA’s Council on Scientific Affairs

Administers Standards Committees Issues Status Reports Run Complaint Programs Council on Scientific Affairs Runs Acceptance Program Publishes Product Reviews

Administers Standards Committees Issues Status Reports Run Complaint Programs Council on Scientific Affairs Runs Acceptance Program Publishes Product Reviews

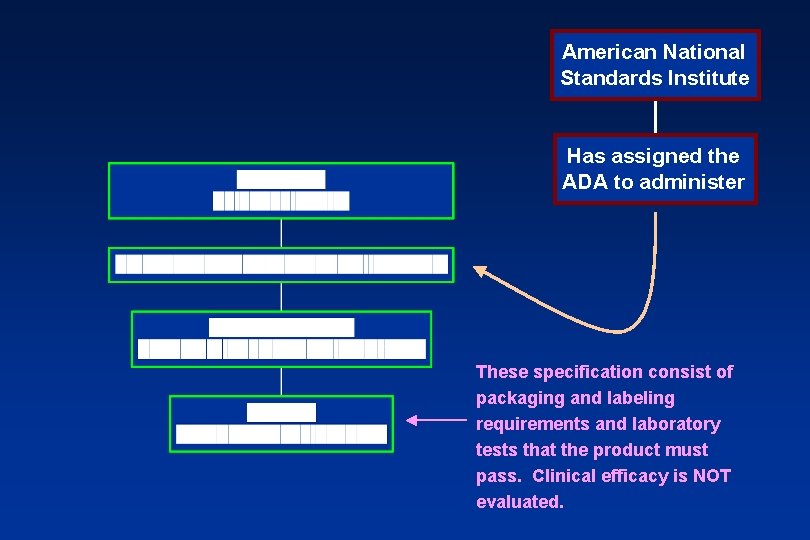
American National Standards Institute Has assigned the ADA to administer These specification consist of packaging and labeling requirements and laboratory tests that the product must pass. Clinical efficacy is NOT evaluated.

Administers Standards Committees Issues Status Reports Run Complaint Programs Council on Scientific Affairs Runs Acceptance Program Publishes Product Reviews

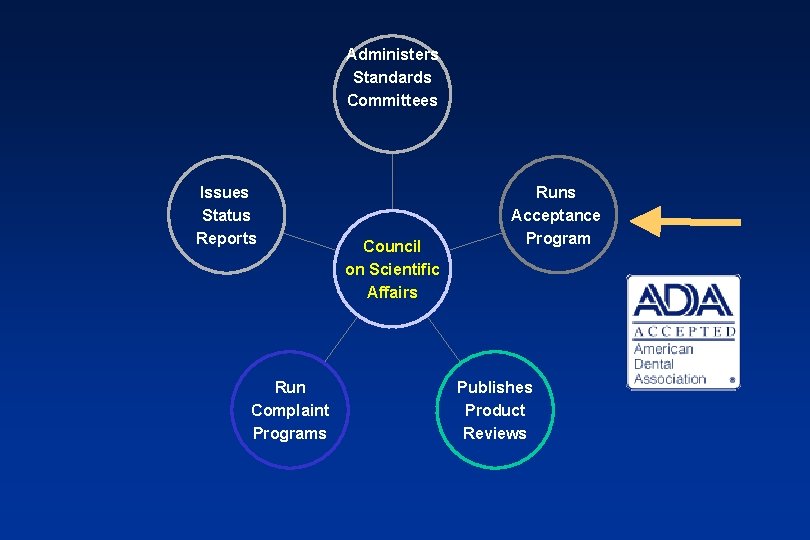
ADA Seal of Acceptance: Covers drugs, therapeutic agents, and materials: • 1300 products currently carry the seal • 30% sold to consumers • 70% sold only to dentists The ADA Seal Program for professional products has been phased out. It ended completely on 12/31/07. The Seal Program on consumer products will continue.

Administers Standards Committees Issues Status Reports Run Complaint Programs Council on Scientific Affairs Runs Acceptance Program Publishes Product Reviews after 2007 no longer covers professional products

ADA Professional Product Review • a quarterly insert that is included with the Journal of the Americans Dental Association • quantitative laboratory data on materials are reported • qualitative practitioner data is reported • replaces acceptance program (seal program), which was increasingly being ignored by both practitioners and manufacturers.

Administers Standards Committees Issues Status Reports Run Complaint Programs Council on Scientific Affairs Runs Acceptance Program Publishes Product Reviews

Administers Standards Committees Issues Status Reports Run Complaint Programs Council on Scientific Affairs Runs Acceptance Program Publishes Product Reviews

U. S. regulation of dental materials: Some dental materials are regulated as “medical devices” by the Food & Drug Administration (FDA). Medical Device Amendments of 1976

U. S. regulation of dental materials: To administer the Medical Device Amendments the FDA: • Set up 19 panels • One panel was the Panel on Review & Classification of Dental Devices

U. S. regulation of dental materials: Three classes of devices: Class I General controls Class II Performance Standards Class III Premarket Approval Unclassified devices are treated as Class II devices

U. S. regulation of dental materials: General controls (Class I): • Ensure compliance with Good Manufacturing practices • Premarket notification (510 K) • Class I: burs, preformed crowns, porcelain teeth

U. S. regulation of dental materials: Performance Standards (Class II): • Ensure compliance with Good Manufacturing practices • Premarket notification (510 K), but includes requirement to demonstrate “substantial equivalence” with existing products • Postmarket surveillance, patient registries • Class II: resin composites, impression materials, calcium hydroxide liners

U. S. regulation of dental materials: Performance Standards (Class II): • “Substantial equivalence” can be demonstrated by compliance with recognized standards • The FDA has recognized 27 SCDP standards and 30 ISO standards

U. S. regulation of dental materials: Premarket Approval (Class III): • Preamendment devices (on the market before 1976) – no premarket approval is required • PMA devices – materials that have a new use or greatly different composition will usually be classified as Class III requiring premarket approval

U. S. regulation of dental materials: Premarket approval involves: • Laboratory tests • Preclinical studies (animal studies) • Clinical evidence of safety and effectiveness (expensive, time consuming)

International standards: • Federation Dentaire Internationale (FDI) – federation made up of national dental associations • Administers dental international standards for the ISO (International Organization for Standardization) • Technical committee in Dentistry – / TC 106 ISO

International standards: ISO / TC 106 • Currently has 145 approved standards (2005, up from 105 in 1999) • Lab tests only (like ADA SCDP standards)

International standards: • CEN (Comité Européen de Normalization); Task Group 55 charged to develop European standards for dental materials, instruments, & equipment • CEN mark – products must have to sell in Europe • CEN usually adopts ISO dental standards NOTE: Unlike U. S. standards, these standards are NOT voluntary. U. S. dentists benefit because manufacturers who want to sell in Europe (just about all manufacturers) must get the CEN mark.
