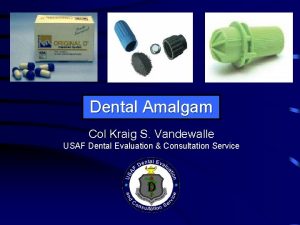
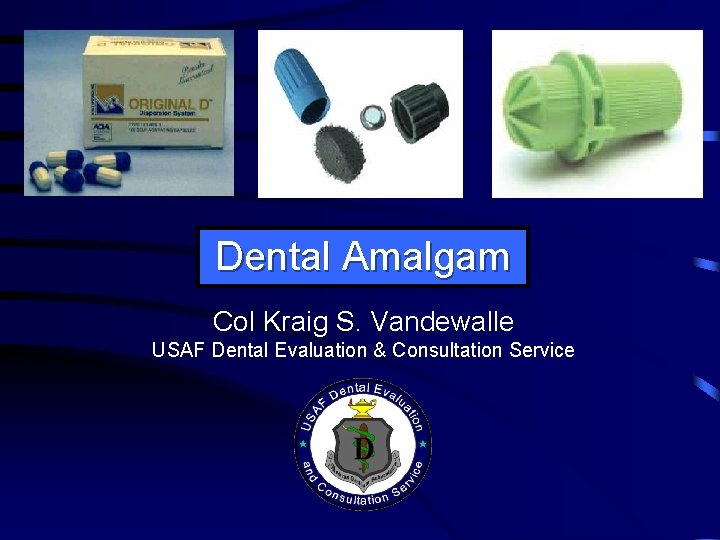
Dental Amalgam Col Kraig S Vandewalle USAF Dental







































- Slides: 59

Dental Amalgam Col Kraig S. Vandewalle USAF Dental Evaluation & Consultation Service

Official Disclaimer • The opinions expressed in this presentation are those of the author and do not necessarily reflect the official position of the US Air Force or the Department of Defense (DOD) • Devices or materials appearing in this presentation are used as examples of currently available products/technologies and do not imply an endorsement by the author and/or the USAF/DOD

Overview • • • History Basic composition Basic setting reactions Classifications Manufacturing Variables in amalgam performance Click here for briefing on dental amalgam (PDF)

History • 1833 – Crawcour brothers introduce amalgam to US • powdered silver coins mixed with mercury – expanded on setting • 1895 – G. V. Black develops formula for modern amalgam alloy • 67% silver, 27% tin, 5% copper, 1% zinc – overcame expansion problems

History • 1960’s – conventional low-copper lathe-cut alloys • smaller particles – first generation high-copper alloys • Dispersalloy (Caulk) – admixture of spherical Ag-Cu eutectic particles with conventional lathe-cut – eliminated gamma-2 phase Mahler J Dent Res 1997

History • 1970’s – first single composition spherical • • Tytin (Kerr) ternary system (silver/tin/copper) • 1980’s – alloys similar to Dispersalloy and Tytin • 1990’s – mercury-free alloys Mahler J Dent Res 1997

Amalgam • An alloy of mercury with another metal.

Why Amalgam? • Inexpensive • Ease of use • Proven track record – >100 years • Familiarity • Resin-free – less allergies than composite Click here for Talking Paper on Amalgam Safety (PDF)

Constituents in Amalgam • Basic – Silver – Tin – Copper – Mercury • Other – Zinc – Indium – Palladium

Basic Constituents • Silver (Ag) – increases strength – increases expansion • Tin (Sn) – decreases expansion – decreased strength – increases setting time Phillip’s Science of Dental Materials 2003

Basic Constituents • Copper (Cu) – ties up tin • reducing gamma-2 formation – increases strength – reduces tarnish and corrosion – reduces creep • reduces marginal deterioration Phillip’s Science of Dental Materials 2003

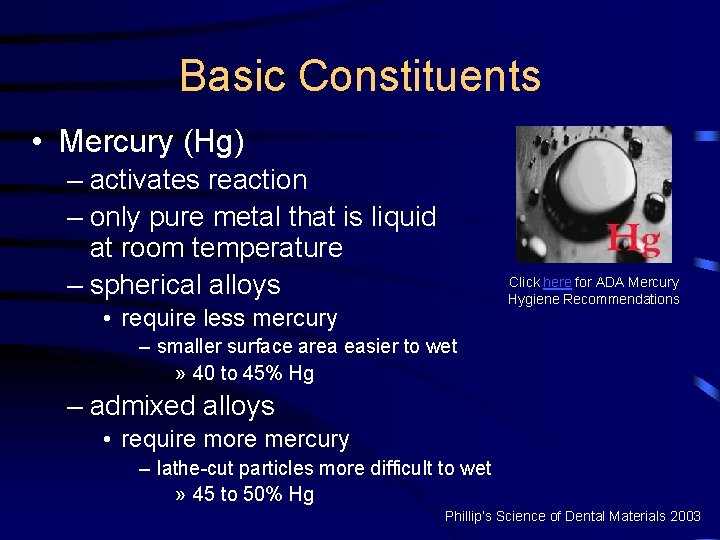
Basic Constituents • Mercury (Hg) – activates reaction – only pure metal that is liquid at room temperature – spherical alloys Click here for ADA Mercury Hygiene Recommendations • require less mercury – smaller surface area easier to wet » 40 to 45% Hg – admixed alloys • require more mercury – lathe-cut particles more difficult to wet » 45 to 50% Hg Phillip’s Science of Dental Materials 2003

Other Constituents • Zinc (Zn) – used in manufacturing • decreases oxidation of other elements – sacrificial anode – provides better clinical performance • less marginal breakdown – Osborne JW Am J Dent 1992 – causes delayed expansion with low Cu alloys • if contaminated with moisture during condensation – Phillips RW JADA 1954 H 2 O + Zn Þ Zn. O + H 2 Phillip’s Science of Dental Materials 2003

Other Constituents • Indium (In) – decreases surface tension • reduces amount of mercury necessary • reduces emitted mercury vapor – reduces creep and marginal breakdown – increases strength – must be used in admixed alloys – example • Indisperse (Indisperse Distributing Company) – 5% indium Powell J Dent Res 1989

Other Constituents • Palladium (Pd) – reduced corrosion – greater luster – example • Valiant Ph. D (Ivoclar Vivadent) – 0. 5% palladium Mahler J Dent Res 1990

Basic Composition • A silver-mercury matrix containing filler particles of silver-tin • Filler (bricks) – Ag 3 Sn called gamma • can be in various shapes – irregular (lathe-cut), spherical, or a combination • Matrix – Ag 2 Hg 3 called gamma 1 • – cement Sn 8 Hg called gamma 2 • voids Phillip’s Science of Dental Materials 2003

Basic Setting Reactions • Conventional low-copper alloys • Admixed high-copper alloys • Single composition high-copper alloys

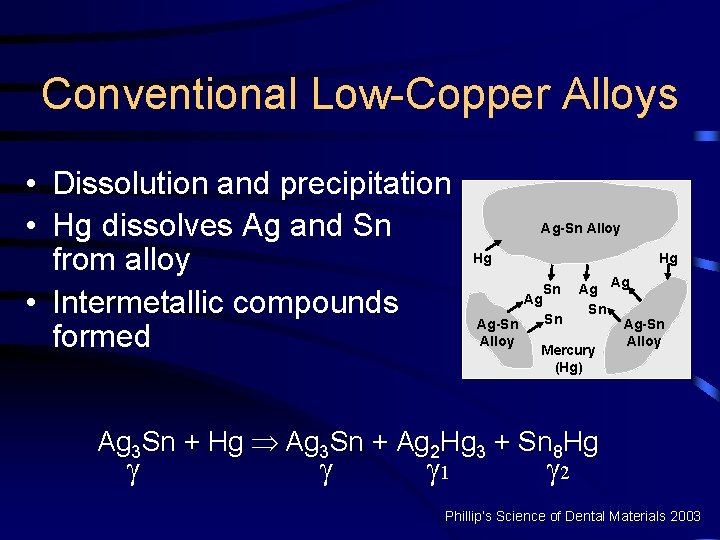
Conventional Low-Copper Alloys • Dissolution and precipitation • Hg dissolves Ag and Sn from alloy • Intermetallic compounds formed Ag-Sn Alloy Hg Hg Ag Ag Ag Sn Sn Ag-Sn Alloy Mercury (Hg) Sn Ag 3 Sn + Hg Þ Ag 3 Sn + Ag 2 Hg 3 + Sn 8 Hg 1 2 Phillip’s Science of Dental Materials 2003

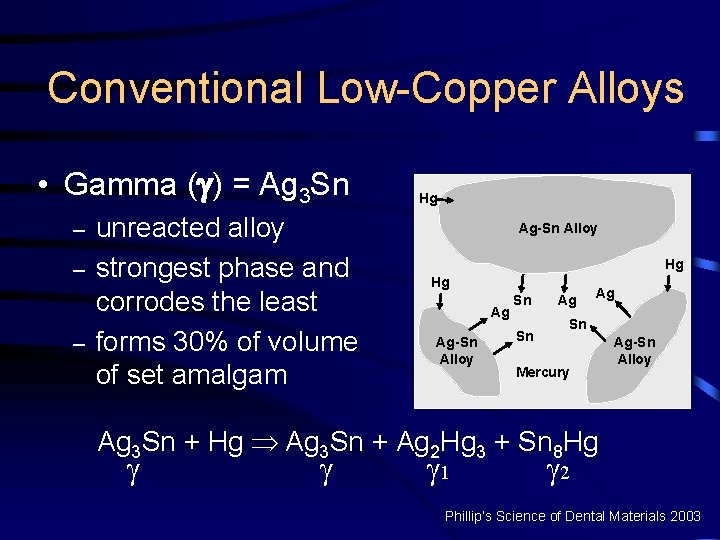
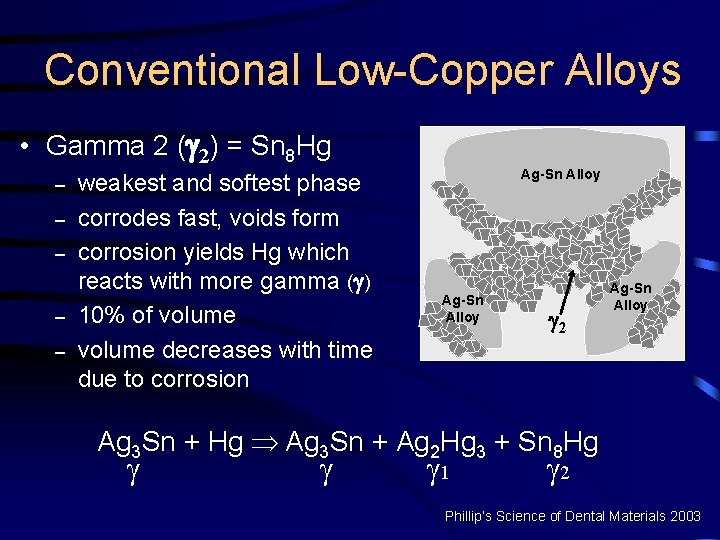
Conventional Low-Copper Alloys • Gamma ( ) = Ag 3 Sn – – – unreacted alloy strongest phase and corrodes the least forms 30% of volume of set amalgam Hg Ag-Sn Alloy Hg Hg Ag Ag-Sn Alloy Sn Sn Ag Ag Sn Mercury Ag-Sn Alloy Ag 3 Sn + Hg Þ Ag 3 Sn + Ag 2 Hg 3 + Sn 8 Hg 1 2 Phillip’s Science of Dental Materials 2003

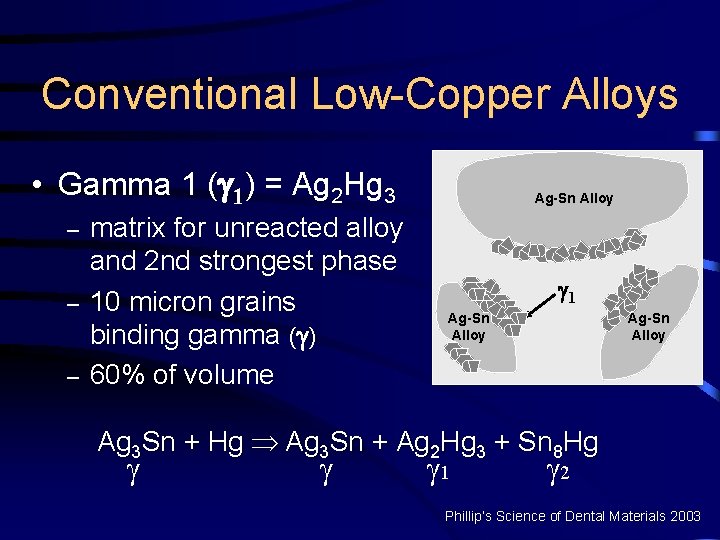
Conventional Low-Copper Alloys • Gamma 1 ( 1) = Ag 2 Hg 3 – – – matrix for unreacted alloy and 2 nd strongest phase 10 micron grains binding gamma ( ) 60% of volume Ag-Sn Alloy 1 Ag-Sn Alloy Ag 3 Sn + Hg Þ Ag 3 Sn + Ag 2 Hg 3 + Sn 8 Hg 1 2 Phillip’s Science of Dental Materials 2003

Conventional Low-Copper Alloys • Gamma 2 ( 2) = Sn 8 Hg – – – weakest and softest phase corrodes fast, voids form corrosion yields Hg which reacts with more gamma ( ) 10% of volume decreases with time due to corrosion Ag-Sn Alloy 2 Ag-Sn Alloy Ag 3 Sn + Hg Þ Ag 3 Sn + Ag 2 Hg 3 + Sn 8 Hg 1 2 Phillip’s Science of Dental Materials 2003

Admixed High-Copper Alloys • Ag enters Hg from Ag-Cu spherical eutectic particles – Ag-Cu Alloy eutectic • an alloy in which the elements are completely soluble in liquid solution but separate into distinct areas upon solidification • Both Ag and Sn enter Hg from Ag 3 Sn particles Hg Ag Ag-Sn Alloy Sn Hg Ag Sn Mercury Ag-Sn Alloy Ag 3 Sn + Ag-Cu + Hg Þ Ag 3 Sn + Ag-Cu + Ag 2 Hg 3 + Cu 6 Sn 5 1 Phillip’s Science of Dental Materials 2003

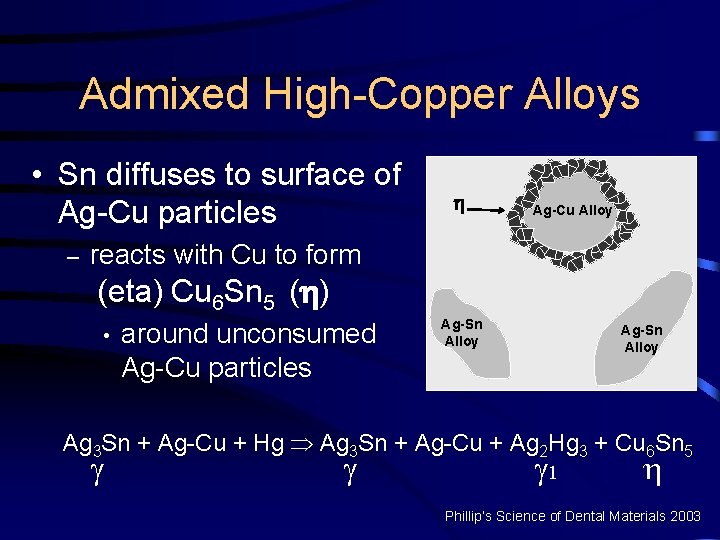
Admixed High-Copper Alloys • Sn diffuses to surface of Ag-Cu particles – Ag-Cu Alloy reacts with Cu to form (eta) Cu 6 Sn 5 ( ) • around unconsumed Ag-Cu particles Ag-Sn Alloy Ag 3 Sn + Ag-Cu + Hg Þ Ag 3 Sn + Ag-Cu + Ag 2 Hg 3 + Cu 6 Sn 5 1 Phillip’s Science of Dental Materials 2003

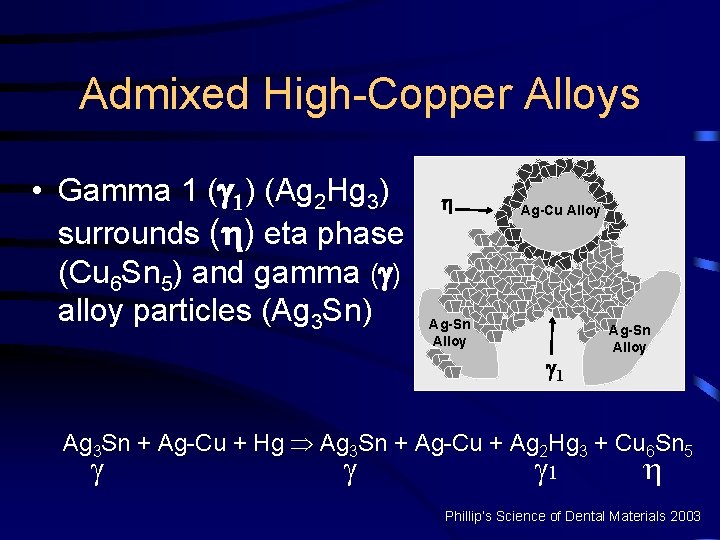
Admixed High-Copper Alloys • Gamma 1 ( 1) (Ag 2 Hg 3) surrounds ( ) eta phase (Cu 6 Sn 5) and gamma ( ) alloy particles (Ag 3 Sn) Ag-Cu Alloy Ag-Sn Alloy 1 Ag-Sn Alloy Ag 3 Sn + Ag-Cu + Hg Þ Ag 3 Sn + Ag-Cu + Ag 2 Hg 3 + Cu 6 Sn 5 1 Phillip’s Science of Dental Materials 2003

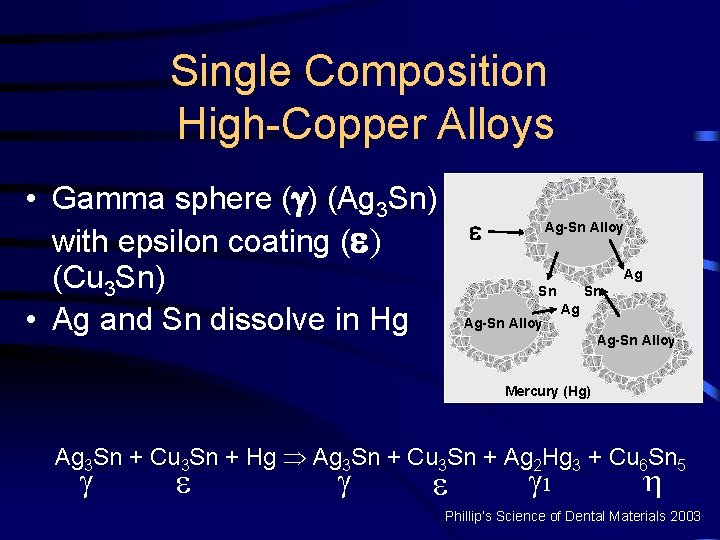
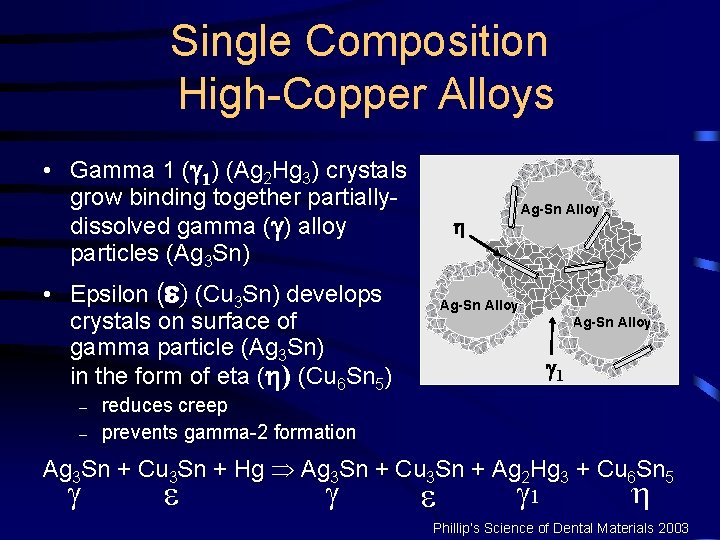
Single Composition High-Copper Alloys • Gamma sphere ( ) (Ag 3 Sn) Ag-Sn Alloy with epsilon coating ( ) Ag (Cu 3 Sn) Sn Sn Ag Ag-Sn Alloy • Ag and Sn dissolve in Hg Ag-Sn Alloy Mercury (Hg) Ag 3 Sn + Cu 3 Sn + Hg Þ Ag 3 Sn + Cu 3 Sn + Ag 2 Hg 3 + Cu 6 Sn 5 1 Phillip’s Science of Dental Materials 2003

Single Composition High-Copper Alloys • Gamma 1 ( 1) (Ag 2 Hg 3) crystals grow binding together partiallydissolved gamma ( ) alloy particles (Ag 3 Sn) • Epsilon ( ) (Cu 3 Sn) develops crystals on surface of gamma particle (Ag 3 Sn) in the form of eta ( ) (Cu 6 Sn 5) – – Ag-Sn Alloy 1 reduces creep prevents gamma-2 formation Ag 3 Sn + Cu 3 Sn + Hg Þ Ag 3 Sn + Cu 3 Sn + Ag 2 Hg 3 + Cu 6 Sn 5 1 Phillip’s Science of Dental Materials 2003

Classifications • Based on copper content • Based on particle shape • Based on method of adding copper

Copper Content • Low-copper alloys – 4 to 6% Cu • High-copper alloys – thought that 6% Cu was maximum amount • due to fear of excessive corrosion and expansion – Now contain 9 to 30% Cu • at expense of Ag Phillip’s Science of Dental Materials 2003

Particle Shape • Lathe cut – low Cu • – New True Dentalloy high Cu • ANA 2000 • Admixture – high Cu • Dispersalloy, Valiant Ph. D • Spherical – low Cu • – Cavex SF high Cu • Tytin, Valiant

Method of Adding Copper • • Single Composition Lathe-Cut (SCL) Single Composition Spherical (SCS) Admixture: Lathe-cut + Spherical Eutectic (ALE) Admixture: Lathe-cut + Single Composition Spherical (ALSCS)

Single Composition Lathe-Cut (SCL) • More Hg needed than spherical alloys • High condensation force needed due to lathe cut • 20% Cu • Example – ANA 2000 (Nordiska Dental)

Single Composition Spherical (SCS) • Spherical particles wet easier with Hg – less Hg needed (42%) • Less condensation force, larger condenser • Gamma particles as 20 micron spheres – with epsilon layer on surface • Examples – – Tytin (Kerr) Valiant (Ivoclar Vivadent)

Admixture: Lathe-cut + Spherical Eutectic (ALE) • Composition – – – 2/3 conventional lathe cut (3% Cu) 1/3 high Cu spherical eutectic (28% Cu) overall 12% Cu, 1% Zn • Initial reaction produces gamma 2 – no gamma 2 within two years • Example – Dispersalloy (Caulk)

Admixture: Lathe-cut + Single Composition Spherical (ALSCS) • High Cu in both lathe-cut and spherical components – 19% Cu • Epsilon layer forms on both components • 0. 5% palladium added – reinforce grain boundaries on gamma 1 • Example – Valiant Ph. D (Ivoclar Vivadent)

Manufacturing Process • Lathe-cut alloys – – Ag & Sn melted together alloy cooled • – heat treat • – – phases solidify 400 ºC for 8 hours grind, then mill to 25 - 50 microns heat treat to release stresses of grinding Phillip’s Science of Dental Materials 2003

Manufacturing Process • Spherical alloys – – melt alloy atomize • – spheres form as particles cool sizes range from 5 - 40 microns • variety improves condensability Phillip’s Science of Dental Materials 2003

Material-Related Variables • • Dimensional change Strength Corrosion Creep

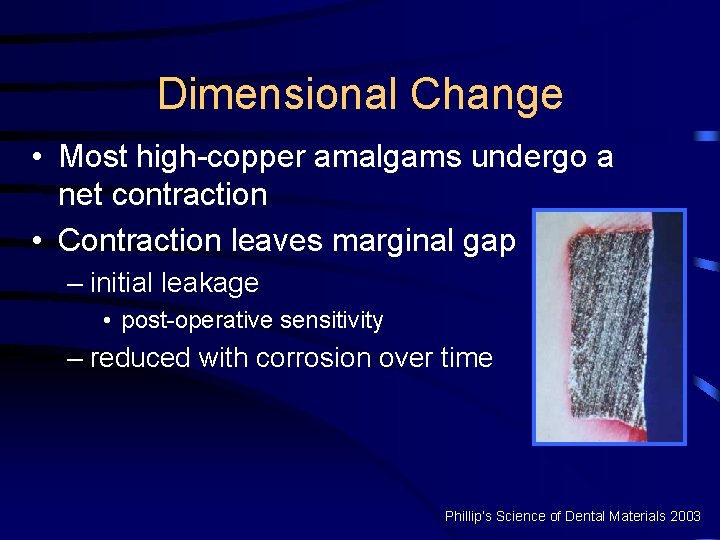
Dimensional Change • Most high-copper amalgams undergo a net contraction • Contraction leaves marginal gap – initial leakage • post-operative sensitivity – reduced with corrosion over time Phillip’s Science of Dental Materials 2003

Dimensional Change • Net contraction – type of alloy • spherical alloys have more contraction – less mercury – condensation technique • greater condensation = higher contraction – trituration time • overtrituration causes higher contraction Phillip’s Science of Dental Materials 2003

Strength • Develops slowly – 1 hr: 40 to 60% of maximum – 24 hrs: 90% of maximum • Spherical alloys strengthen faster – require less mercury • Higher compressive vs. tensile strength • Weak in thin sections – unsupported edges fracture Phillip’s Science of Dental Materials 2003

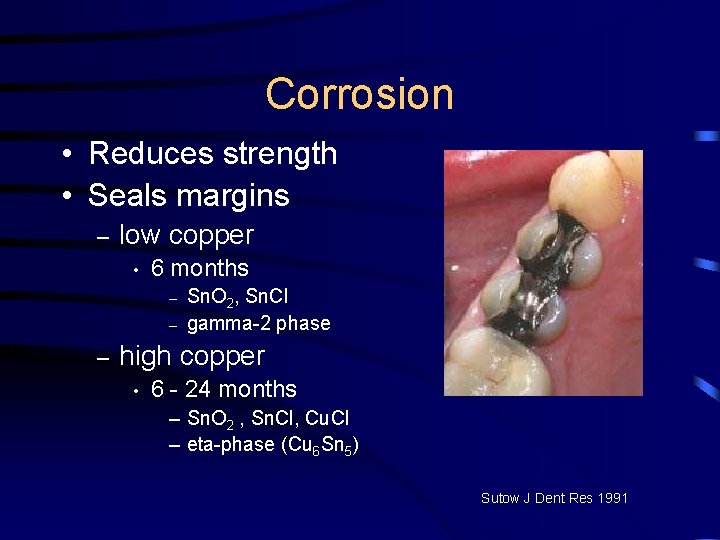
Corrosion • Reduces strength • Seals margins – low copper • 6 months – – – Sn. O 2, Sn. Cl gamma-2 phase high copper • 6 - 24 months – Sn. O 2 , Sn. Cl, Cu. Cl – eta-phase (Cu 6 Sn 5) Sutow J Dent Res 1991

Creep • Slow deformation of amalgam placed under a constant load – • load less than that necessary to produce fracture Gamma 2 dramatically affects creep rate – slow strain rates produces plastic deformation • allows gamma-1 grains to slide • Correlates with marginal breakdown Phillip’s Science of Dental Materials 2003

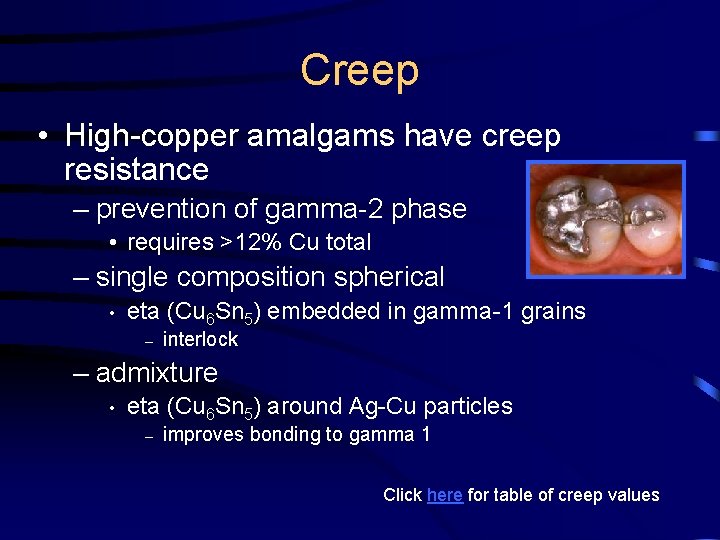
Creep • High-copper amalgams have creep resistance – prevention of gamma-2 phase • requires >12% Cu total – single composition spherical • eta (Cu 6 Sn 5) embedded in gamma-1 grains – interlock – admixture • eta (Cu 6 Sn 5) around Ag-Cu particles – improves bonding to gamma 1 Click here for table of creep values

Dentist-Controlled Variables • Manipulation – – trituration condensation burnishing polishing

Trituration • Mixing time – refer to manufacturer recommendations • Click here for details • Overtrituration – “hot” mix • – – sticks to capsule decreases working / setting time slight increase in setting contraction • Undertrituration – grainy, crumbly mix Phillip’s Science of Dental Materials 2003

Condensation • Forces – lathe-cut alloys • small condensers • high force – spherical alloys • large condensers • less sensitive to amount of force • vertical / lateral with vibratory motion – admixture alloys • intermediate handling between lathe-cut and spherical

Burnishing • Pre-carve – removes excess mercury – improves margin adaptation • Post-carve – improves smoothness • Combined – less leakage Ben-Amar Dent Mater 1987

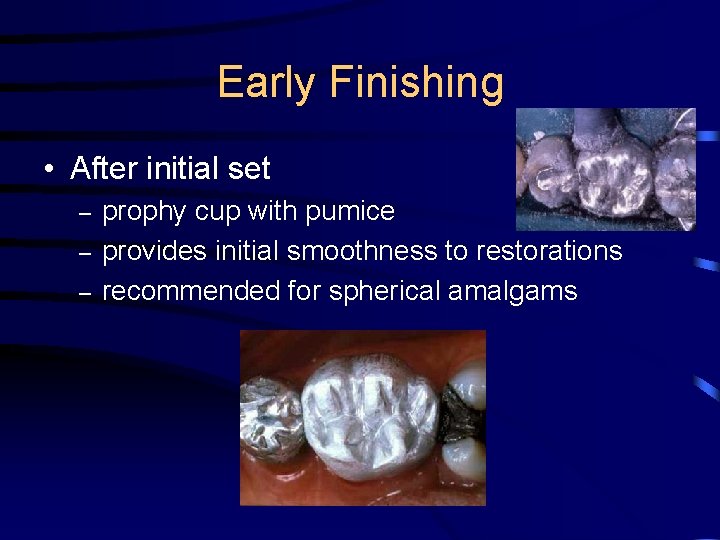
Early Finishing • After initial set – – – prophy cup with pumice provides initial smoothness to restorations recommended for spherical amalgams

Polishing • • Increased smoothness Decreased plaque retention Decreased corrosion Clinically effective? – no improvement in marginal integrity • Mayhew Oper Dent 1986 • Collins J Dent 1992 – Click here for abstract

Alloy Selection • Handling characteristics • Mechanical and physical properties • Clinical performance Click here for more details

Handling Characteristics • Spherical – advantages • easier to condense – around pins • hardens rapidly • smoother polish – disadvantages • difficult to achieve tight contacts • higher tendency for overhangs Phillip’s Science of Dental Materials 2003

Handling Characteristics • Admixed – advantages • easy to achieve tight contacts • good polish – disadvantages • hardens slowly – lower early strength

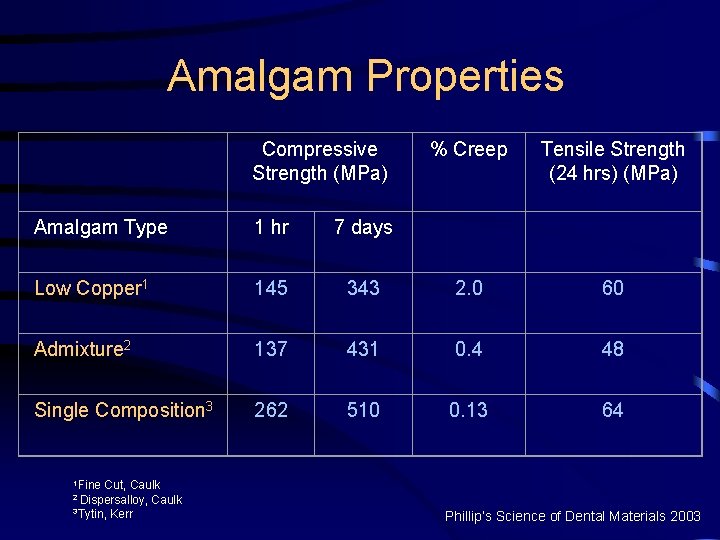
Amalgam Properties Compressive Strength (MPa) % Creep Tensile Strength (24 hrs) (MPa) Amalgam Type 1 hr 7 days Low Copper 1 145 343 2. 0 60 Admixture 2 137 431 0. 4 48 Single Composition 3 262 510 0. 13 64 1 Fine Cut, Caulk 3 Tytin, Kerr 2 Dispersalloy, Phillip’s Science of Dental Materials 2003

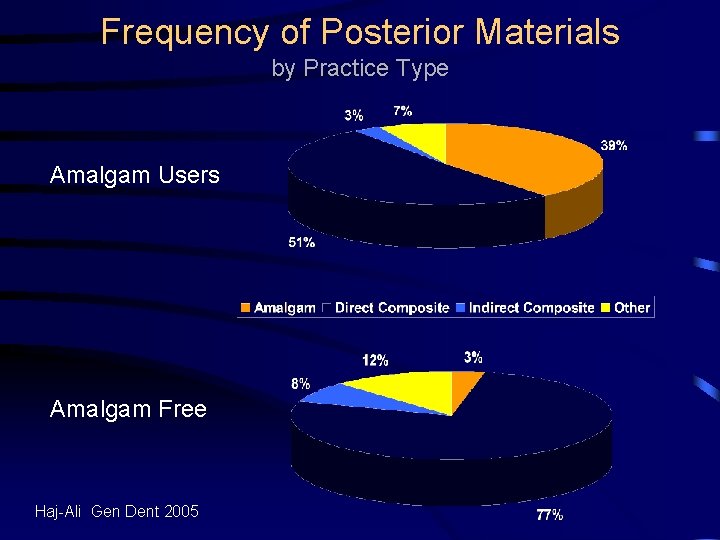
Survey of Practice Types Civilian General Dentists Amalgam Free Amalgam Users Haj-Ali Gen Dent 2005

Frequency of Posterior Materials by Practice Type Amalgam Users Amalgam Free Haj-Ali Gen Dent 2005

Profile of Amalgam Users Civilian Practitioners Do you use amalgam in your practice? No Do you place fewer amalgams than 5 years ago? No Yes DPR 2005

Review of Clinical Studies (Failure Rates in Posterior Permanent Teeth) % Annual Failure Hickel J Adhes Dent 2001

Review of Clinical Studies (Failure Rates in Posterior Permanent Teeth) % Annual Failure Standard Deviation Longitudinal and Cross-Sectional Data Manhart Oper Dent 2004 Click here for abstract

Acknowledgements • Dr. David Charlton • Dr. Charles Hermesch • Col Salvador Flores Questions/Comments Col Kraig Vandewalle – DSN 792 -7670 – ksvandewalle@nidbr. med. navy. mil
 Caracol col col sal de tu casita
Caracol col col sal de tu casita Kraig brockschmidt
Kraig brockschmidt Topic
Topic What is solvent in science grade 7
What is solvent in science grade 7 Separations afi
Separations afi Usaf 2 line prf examples
Usaf 2 line prf examples Progressive discipline afi
Progressive discipline afi Afi 36 2502
Afi 36 2502 Usaf first sergeant academy
Usaf first sergeant academy Afi 36-2908 family care plans
Afi 36-2908 family care plans Air force progressive discipline chart
Air force progressive discipline chart Hs ratti
Hs ratti Colonel matt hepburn
Colonel matt hepburn Col 3 nasb
Col 3 nasb Create fulltext index
Create fulltext index Col john o ensor middle school
Col john o ensor middle school Cancer du col
Cancer du col Catastro multiproposito
Catastro multiproposito Dr nouira urologue
Dr nouira urologue Col 1:21-23
Col 1:21-23 Macro anatomy of gingiva
Macro anatomy of gingiva Frank skimmerhorn
Frank skimmerhorn Col 106 amit kumar
Col 106 amit kumar Distocia dinamica
Distocia dinamica Col 106 amit kumar
Col 106 amit kumar Cól miera
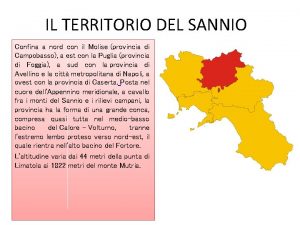
Cól miera Confinava con il sannio
Confinava con il sannio Douglas mcgregor
Douglas mcgregor 1/2 col
1/2 col Move zoppa gentil piede ineguale
Move zoppa gentil piede ineguale Coach col
Coach col Aisha mian leaked
Aisha mian leaked G.col/managewallet
G.col/managewallet Colossians 1 9-14
Colossians 1 9-14 Ntu col
Ntu col Cancer du col
Cancer du col Col 16
Col 16 Rüzgarların oluşturduğu yer şekilleri
Rüzgarların oluşturduğu yer şekilleri Col
Col Adenomectomie transvezicala
Adenomectomie transvezicala Colossians 1 15-18
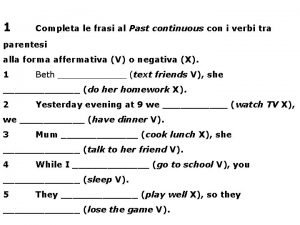
Colossians 1 15-18 Frasi con il present continuous
Frasi con il present continuous Mendian larrartean
Mendian larrartean Col legi parroquial don jose lluch
Col legi parroquial don jose lluch Dünyadaki tatlı su rezerv alanları
Dünyadaki tatlı su rezerv alanları Col legi pius xii
Col legi pius xii Verdura col
Verdura col Agricultura 79 col escandon
Agricultura 79 col escandon Colossians 3 12 13
Colossians 3 12 13 2 corinthians 4
2 corinthians 4 Entamoeba col
Entamoeba col Col 106 amit kumar
Col 106 amit kumar Karasal çöller
Karasal çöller Red hot root words
Red hot root words Col 106 amit kumar
Col 106 amit kumar Modifikasyon
Modifikasyon Kolonya çözücü ve çözüneni
Kolonya çözücü ve çözüneni Contraindications of composite restoration
Contraindications of composite restoration Dental inlay definition
Dental inlay definition What is cavity varnish
What is cavity varnish