Density What is density How can we measure









- Slides: 11

Density What is density? How can we measure it?

Density n n n The amount of matter in a given volume. Density = Mass/Volume Is a property of that substance. Most materials are densest as a solid, EXCEPT WATER.

Phases of Matter n n Matter can be either Solid, Liquid or Gas. Most materials are densest as a SOLID, EXCEPT water which is most dense at 40 C (liquid).

Density of Water n n Density of liquid water is 1. 0 g/L Liquid water is the densest state. ICE WATER STEAM

Densities in Water n n n Less dense then water floats Same density as water stays suspended- (not floating not sinking) More dense then water sinks

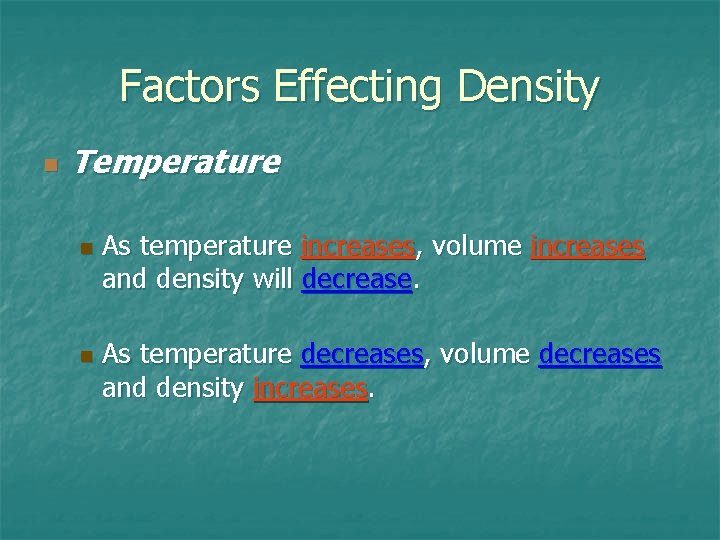
Factors Effecting Density n Temperature n n As temperature increases, volume increases and density will decrease. As temperature decreases, volume decreases and density increases.

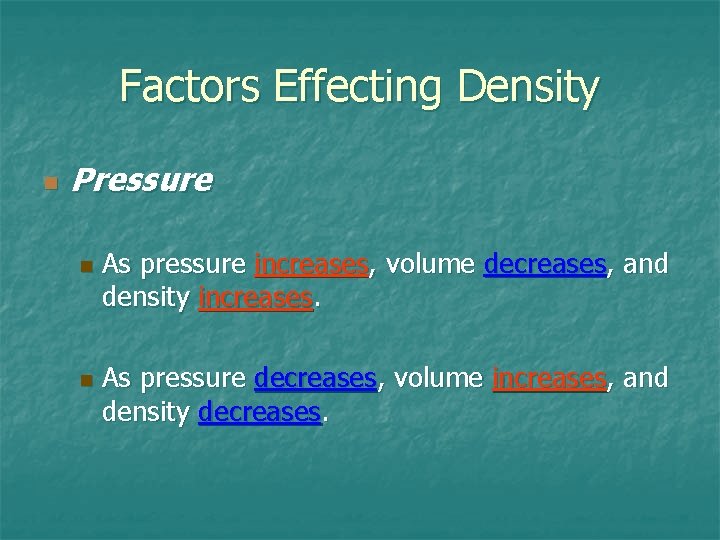
Factors Effecting Density n Pressure n n As pressure increases, volume decreases, and density increases. As pressure decreases, volume increases, and density decreases.

Determining Densities Density = Mass/ Volume Solve the following problems using the formula: 1. 2. An object displaces 12. 2 m. L of water and has a mass of 230. 0 g. What is the density of the object? An object has a density of 15 g/cm 3 and a mass of 3 g. What is it’s volume?

ANSWERS 1. ) D = M/V n 2. ) D = M/V n 15 g/cm 3 = 3 g/V D = 230. 0 g / 12. 2 m. L n n D = 18. 85 g/m. L 15 g/cm 3 = 3 g 1 V n (15 g/cm 3)V = 3 g n 3 g/ 15 g/cm 3 = V n V = 0. 2 cm 3

Quiz n n n Number your paper 1 -6 and answer the questions on the following slide. You may use your notes!! You will have 10 minutes to complete this quiz. Good Luck! DONT FORGET TO PUT YOUR NAME ON THE PAPER!!

Quiz Questions – NO TALKING 1. 2. 3. 4. 5. 6. What is the formula for density? At which temperature and state is water most dense? A substance that is sinking in water is more or less dense then water? What two factors effect density? As the temperature of an object increases what happens to volume and density? An object displaces 5. 4 m. L of water and has a mass of 540. 0 g. What is the density of the object? Please turn your paper over when you are done.