Density What is Density Density The amount of








- Slides: 15

Density

What is Density? �Density: The amount of matter that occupies a certain space. Densities of Common Elements and Compounds Substance Density grams per m. L or cc Water 1. 00 Aluminum, Al 2. 70 Iron, Fe 7. 80 Gold, Au 19. 30 Mercury, Hg 13. 5 � It is the mass per unit volume of a substance.

Let’s look at Examples of Density: Lead is much more dense than iron. So given the same volume of each, lead will weigh more (have more mass). 2. Ice (solid) is less dense than water (liquid). Therefore ice floats on water 1. Frozen Ice Liquid Water

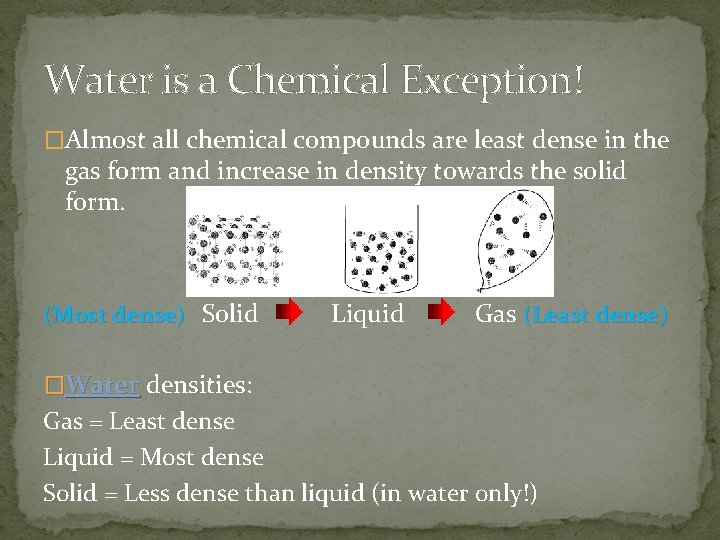
Water is a Chemical Exception! �Almost all chemical compounds are least dense in the gas form and increase in density towards the solid form. (Most dense) Solid Liquid Gas (Least dense) �Water densities: Gas = Least dense Liquid = Most dense Solid = Less dense than liquid (in water only!)

Calculating Density �To calculate density you need two things: �Mass and Volume D = density D=M M = mass V V = volume �Can have several different units: �kg/l �g/ml �g/cm 3

Example �A softball has a mass of 360 g and a volume of 270 cm 3. Find its density. Given: Mass = 360 g Volume = 270 cm 3 Required: Density (in g/cm 3) Answer : D = M/V = 360 g / 270 cm 3 = 1. 33 g/cm 3

Displacement �https: //www. youtube. com/watch? v=ijj 58 x. D 5 f. DI

Example �We measured the volume of a piece of copper by displacement in a beaker of water. The initial volume of water was 21 ml and after placing our piece of copper into the beaker, the new volume was 26 ml. Calculate the density this piece of copper weighing 53. 47 g. (remember density = mass / volume) Mass = 53. 47 g Volume = 26 ml-21 ml = 5 ml Density = 53. 47 g = 10. 694 g/ml 5 ml �A pen has what density?

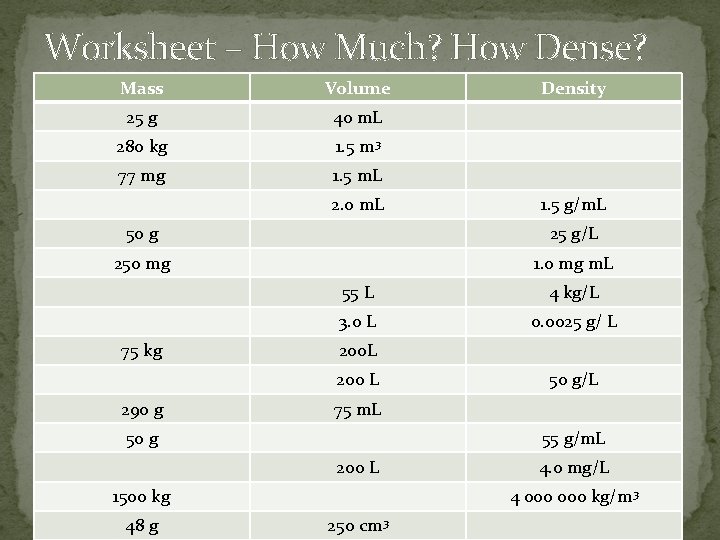
Worksheet – How Much? How Dense? Mass Volume 25 g 40 m. L 280 kg 1. 5 m 3 77 mg 1. 5 m. L 2. 0 m. L Density 1. 5 g/m. L 50 g 25 g/L 250 mg 1. 0 mg m. L 75 kg 55 L 4 kg/L 3. 0 L 0. 0025 g/ L 200 L 290 g 75 m. L 50 g 55 g/m. L 200 L 1500 kg 48 g 50 g/L 4. 0 mg/L 4 000 kg/m 3 250 cm 3

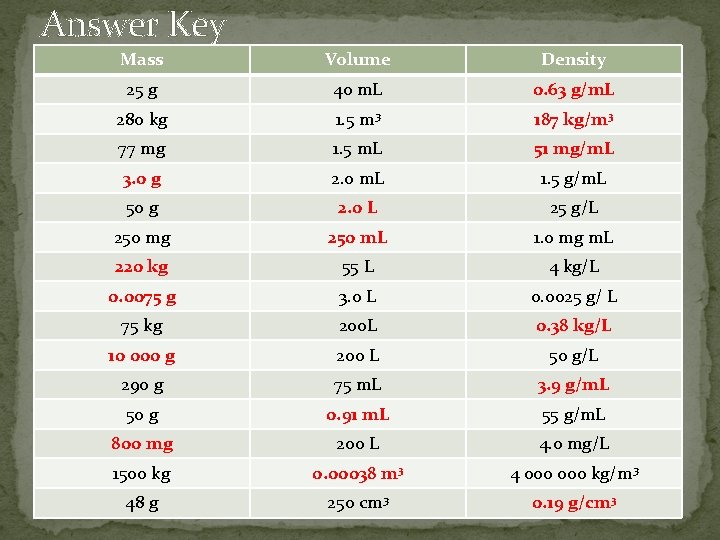
Answer Key Mass Volume Density 25 g 40 m. L 0. 63 g/m. L 280 kg 1. 5 m 3 187 kg/m 3 77 mg 1. 5 m. L 51 mg/m. L 3. 0 g 2. 0 m. L 1. 5 g/m. L 50 g 2. 0 L 25 g/L 250 mg 250 m. L 1. 0 mg m. L 220 kg 55 L 4 kg/L 0. 0075 g 3. 0 L 0. 0025 g/ L 75 kg 200 L 0. 38 kg/L 10 000 g 200 L 50 g/L 290 g 75 m. L 3. 9 g/m. L 50 g 0. 91 m. L 55 g/m. L 800 mg 200 L 4. 0 mg/L 1500 kg 0. 00038 m 3 4 000 kg/m 3 48 g 250 cm 3 0. 19 g/cm 3

Density Activity; It’s Dense! �You are going to determine the density of 6 different objects. (4 provided, 2 you provide) �Step 1: Find the mass of your object using the electronic scale (in grams and to 2 decimal places) �Step 2: We will be using water displacement to calculate volume. � Submerge the object in a graduated cylinder filled with water and note the volume change. � Volume of object = final volume after displacement – initial volume before displacement. �Step 3: Calculate density.

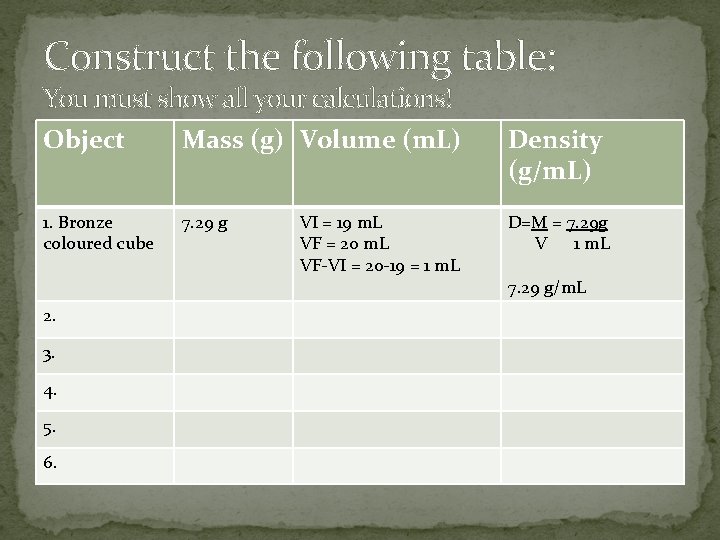
Construct the following table: You must show all your calculations! Object Mass (g) Volume (m. L) Density (g/m. L) 1. Bronze coloured cube 7. 29 g D=M = 7. 29 g V 1 m. L 2. 3. 4. 5. 6. VI = 19 m. L VF = 20 m. L VF-VI = 20 -19 = 1 m. L 7. 29 g/m. L

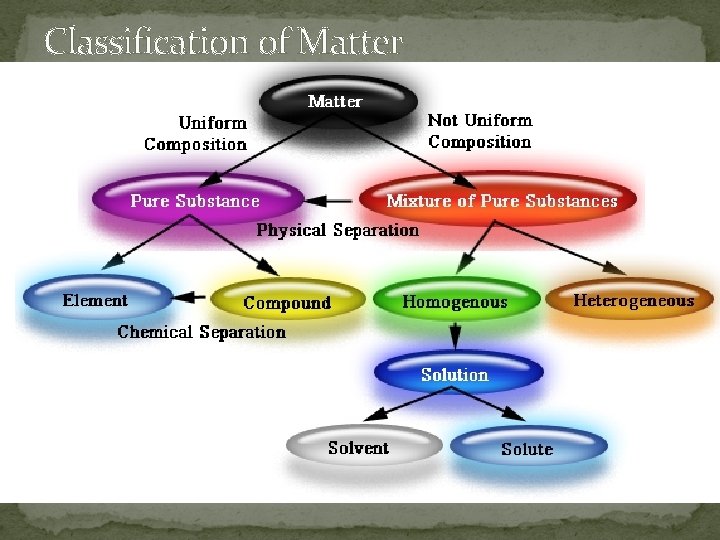
Classification of Matter

Classification of Matter �Matter: Anything that has mass and takes up space. �Mixture: Two or more substances mixed together. �Pure Substance: A substance that contains only one kind of matter. �Mechanical Mixture: A mixture of two or more components that can be seen with the unaided eye.

�Solutions: A homogeneous mixture of two or more substances. �Heterogeneous: Consisting of elements that aren’t all the same (variability). �Homogeneous: Uniform (the same) throughout. �Precipitate: The formation of a solid in a solution during a chemical reaction.