Density sink or float Liquid Layers If you







- Slides: 13
Density: sink or float?

Liquid Layers • If you pour together liquids that don’t mix and have different densities, they will form liquid layers. • The liquid with the highest density will be on the bottom. • The liquid with the lowest density will be on the top.

Sink or Float • Ice floats in water because the density of ice is less than the density of water. • Aluminum sinks because its density is greater than the density of water. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 3

Water, Oil…and a Superball The oil is less dense than the water, so it’s on top. The superball is less dense than water, but more dense than oil, so it sinks to the bottom of the oil layer, yet floats on the top of the water layer.

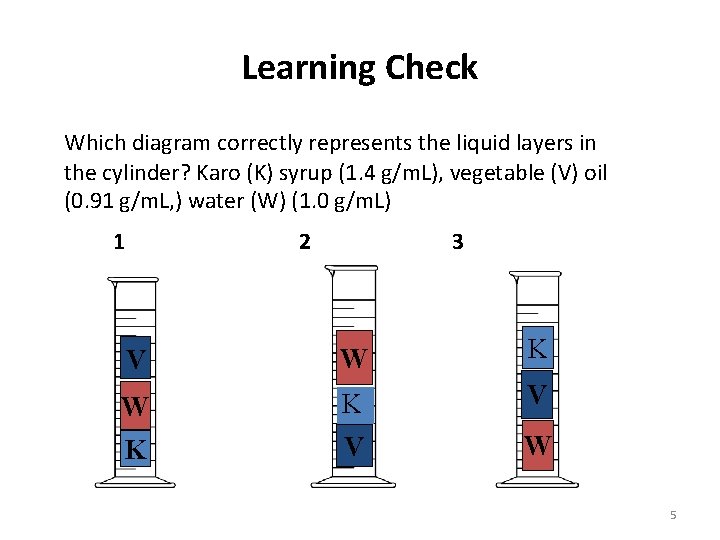
Learning Check Which diagram correctly represents the liquid layers in the cylinder? Karo (K) syrup (1. 4 g/m. L), vegetable (V) oil (0. 91 g/m. L, ) water (W) (1. 0 g/m. L) 1 2 3 V W K V K V W 5

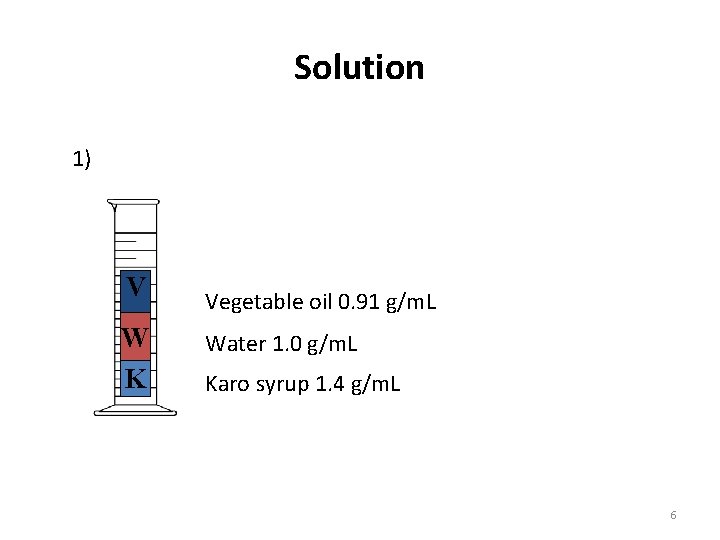
Solution 1) V W K Vegetable oil 0. 91 g/m. L Water 1. 0 g/m. L Karo syrup 1. 4 g/m. L 6

Liquid Layers • Check out this picture from your book. Which layer has the highest density? • Which layer has the lowest density? • Imagine that the liquids have the following densities: – 10 g/cm 3. 3 g/cm 3. – 6 g/cm 3. 5 g/cm 3. • Which number would go with which layer?

Liquid Layers • Check out this picture from your book. Which layer has the highest density? • Which layer has the lowest density? • Imagine that the liquids have the following densities: 10 g/cm 3 – – 6 g/cm 3 3 g/cm 3 5 g/cm 3 • Which number would go with which layer? 3 g/cm 3 5 g/cm 3 6 g/cm 3 10 g/cm 3

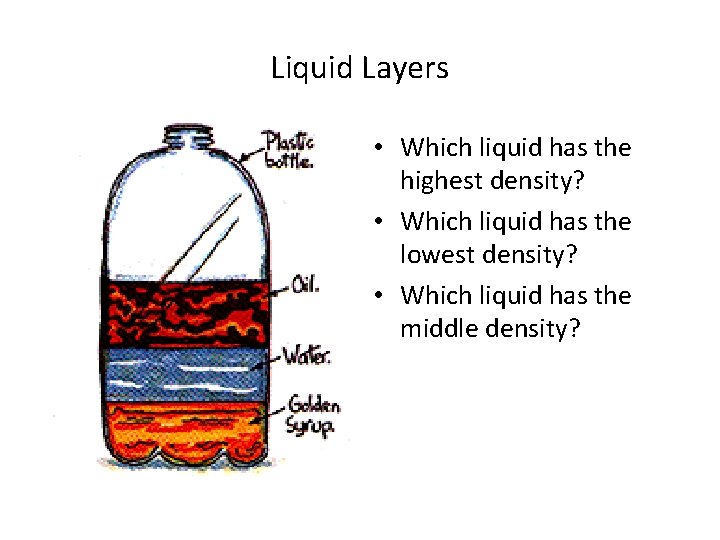
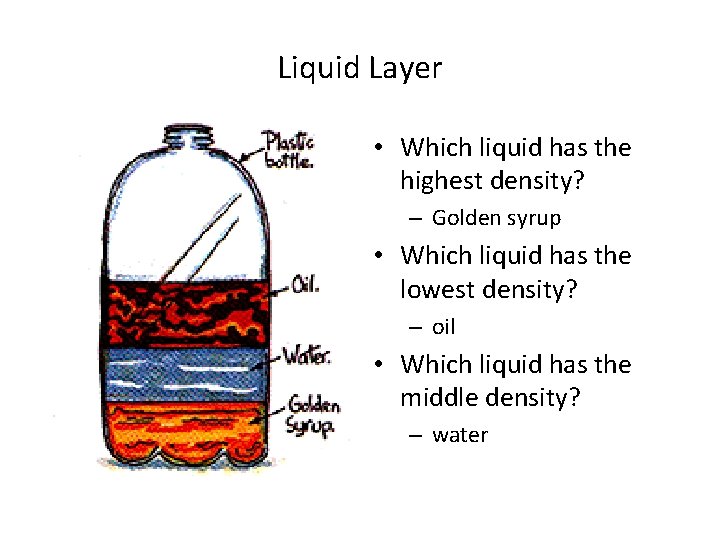
Liquid Layers • Which liquid has the highest density? • Which liquid has the lowest density? • Which liquid has the middle density?

Liquid Layer • Which liquid has the highest density? – Golden syrup • Which liquid has the lowest density? – oil • Which liquid has the middle density? – water

Liquid Layers – Try on your own! • Imagine that the liquids on the right have the following densities: – 15 g/cm 3 – 3 g/cm 3 – 7 g/cm 3 10 g/cm 3 9 g/cm 3 12 g/cm 3 • Match the colors to the correct densities. 3 g/cm 3 7 g/cm 3 9 g/cm 3 10 g/cm 3 12 g/cm 3 15 g/cm 3

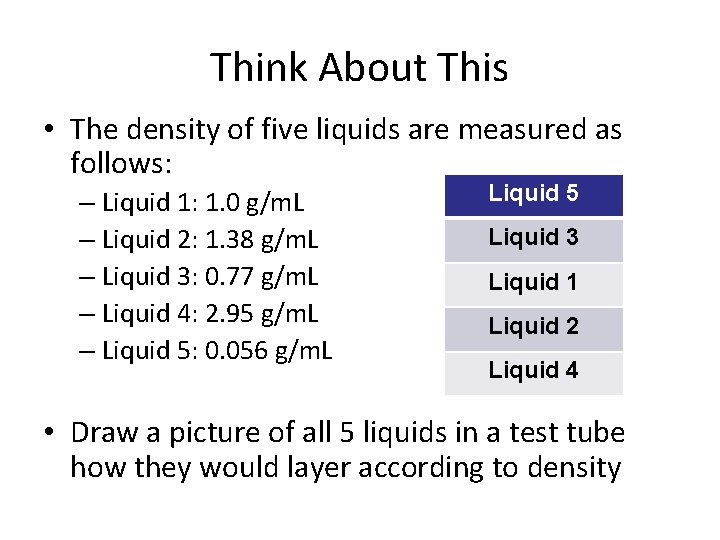
Think About This • The density of five liquids are measured as follows: – Liquid 1: 1. 0 g/m. L – Liquid 2: 1. 38 g/m. L – Liquid 3: 0. 77 g/m. L – Liquid 4: 2. 95 g/m. L – Liquid 5: 0. 056 g/m. L • Draw a picture of all 5 liquids in a test tube how they would layer according to density

Think About This • The density of five liquids are measured as follows: – Liquid 1: 1. 0 g/m. L – Liquid 2: 1. 38 g/m. L – Liquid 3: 0. 77 g/m. L – Liquid 4: 2. 95 g/m. L – Liquid 5: 0. 056 g/m. L Liquid 5 Liquid 3 Liquid 1 Liquid 2 Liquid 4 • Draw a picture of all 5 liquids in a test tube how they would layer according to density