Density ratio Density is a of an objects
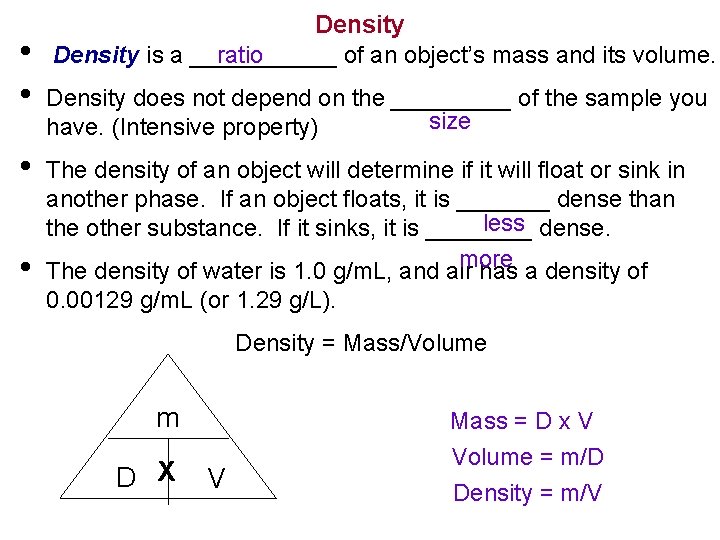
• • Density ratio Density is a ______ of an object’s mass and its volume. Density does not depend on the _____ of the sample you size have. (Intensive property) The density of an object will determine if it will float or sink in another phase. If an object floats, it is _______ dense than less dense. the other substance. If it sinks, it is ____ more The density of water is 1. 0 g/m. L, and air has a density of 0. 00129 g/m. L (or 1. 29 g/L). Density = Mass/Volume m D X V Mass = D x V Volume = m/D Density = m/V

Methods to Find Density 1. Measurement 2. Water Displacement 3. Density Column 4. Solve
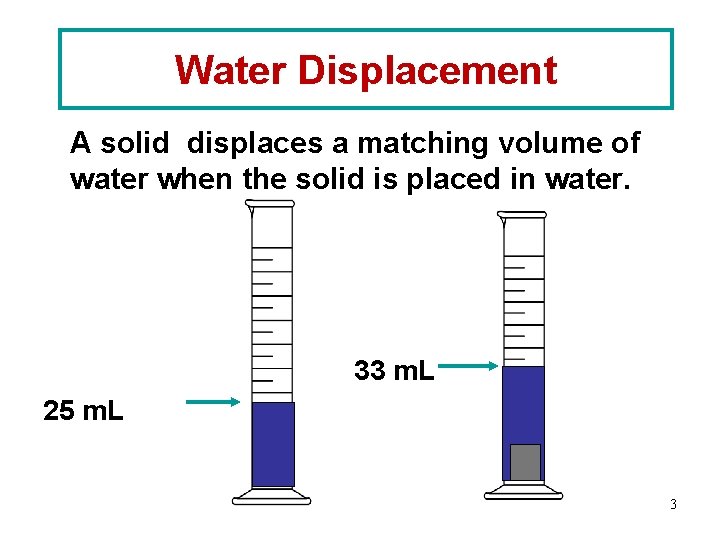
Water Displacement A solid displaces a matching volume of water when the solid is placed in water. 33 m. L 25 m. L 3
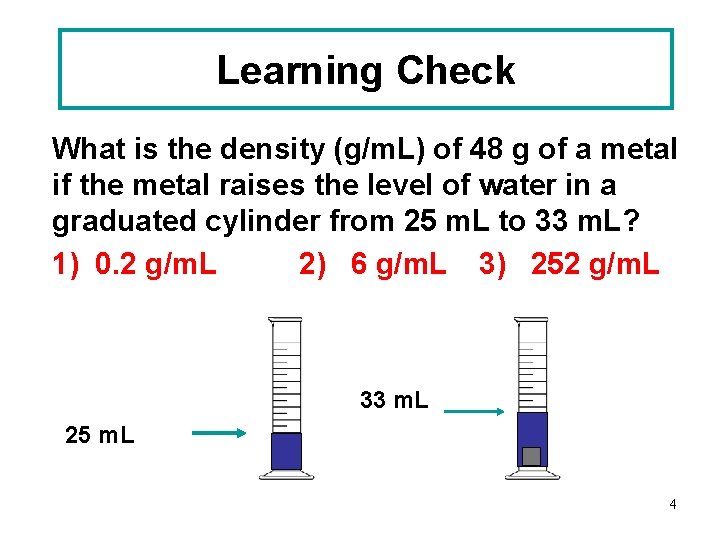
Learning Check What is the density (g/m. L) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? 1) 0. 2 g/m. L 2) 6 g/m. L 3) 252 g/m. L 33 m. L 25 m. L 4
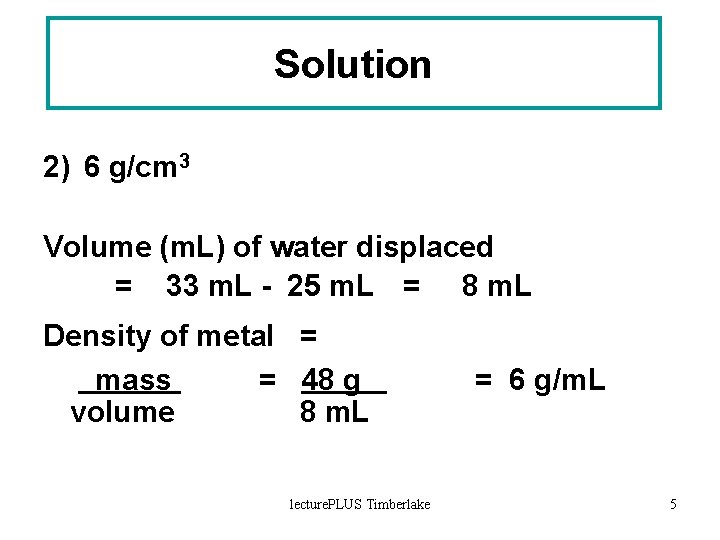
Solution 2) 6 g/cm 3 Volume (m. L) of water displaced = 33 m. L - 25 m. L = 8 m. L Density of metal = mass = 48 g volume 8 m. L lecture. PLUS Timberlake = 6 g/m. L 5
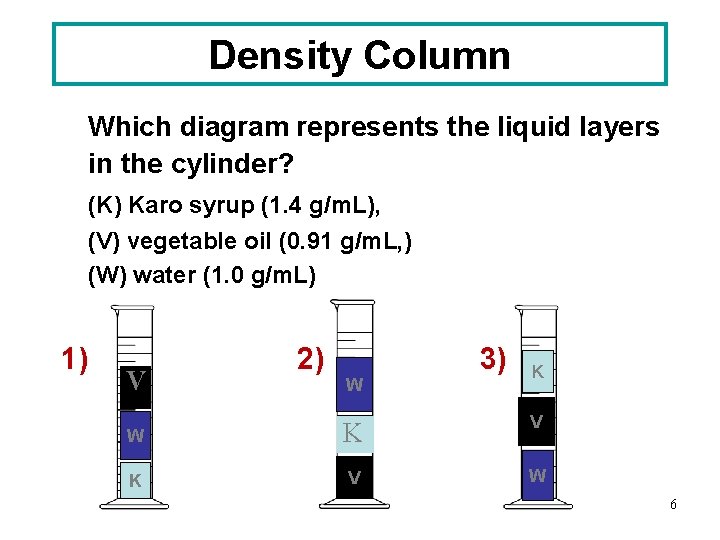
Density Column Which diagram represents the liquid layers in the cylinder? (K) Karo syrup (1. 4 g/m. L), (V) vegetable oil (0. 91 g/m. L, ) (W) water (1. 0 g/m. L) 1) V 2) 3) W K V K V W 6
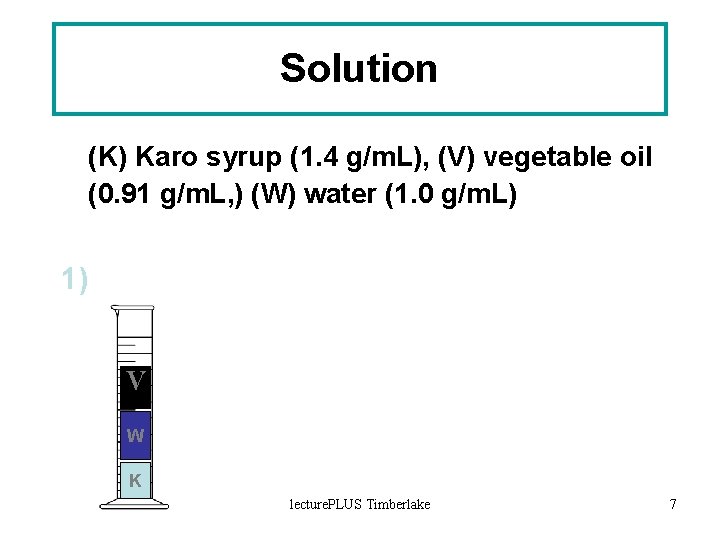
Solution (K) Karo syrup (1. 4 g/m. L), (V) vegetable oil (0. 91 g/m. L, ) (W) water (1. 0 g/m. L) 1) V W K lecture. PLUS Timberlake 7

Density Practice Problems: (1) The density of gold is 19. 3 g/cm 3. How much would the mass of a bar of gold be? Assume a bar of gold has the following dimensions: L= 27 cm W= 9. 0 cm H= 5. 5 cm Steps to solve: 1. Volume = L x W x H Volume = 27 x 9. 0 x 5. 5 = 1336. 5 cm 3 2. Mass = D x V mass = 19. 3 g/cm 3 x 1336. 5 cm 3 = 25, 794. 45 g
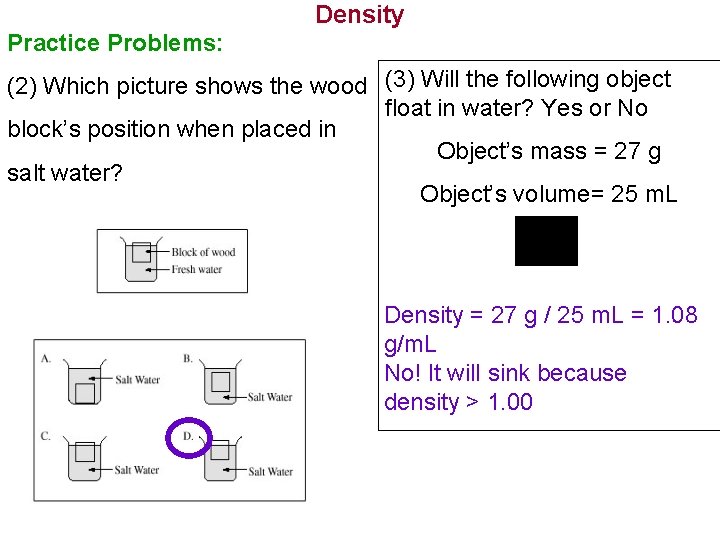
Density Practice Problems: (2) Which picture shows the wood (3) Will the following object float in water? Yes or No block’s position when placed in Object’s mass = 27 g salt water? Object’s volume= 25 m. L Density = 27 g / 25 m. L = 1. 08 g/m. L No! It will sink because density > 1. 00
Density • Density is an INTENSIVE property of matter. - does NOT depend Brick Styrofoam on quantity of matter. - color, melting point, boiling point, odor, density • Contrast with EXTENSIVE - depends on quantity of matter. - mass, volume, heat content (calories)
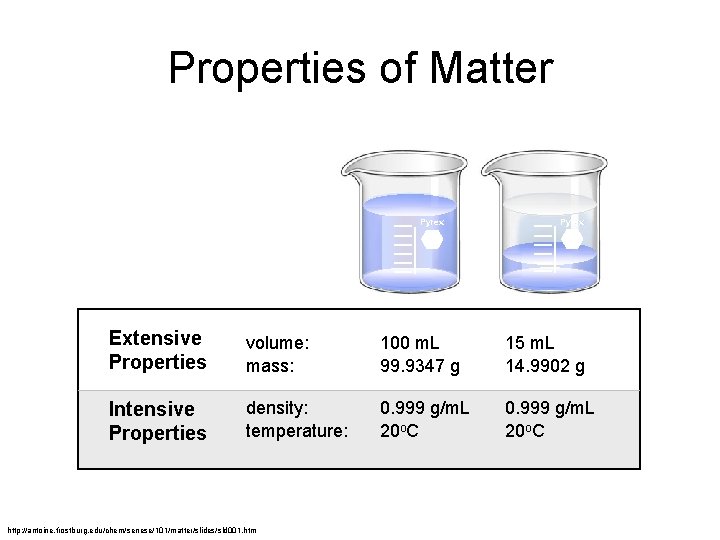
Properties of Matter Pyrex Extensive Properties volume: mass: 100 m. L 99. 9347 g 15 m. L 14. 9902 g Intensive Properties density: temperature: 0. 999 g/m. L 20 o. C http: //antoine. frostburg. edu/chem/senese/101/matter/slides/sld 001. htm
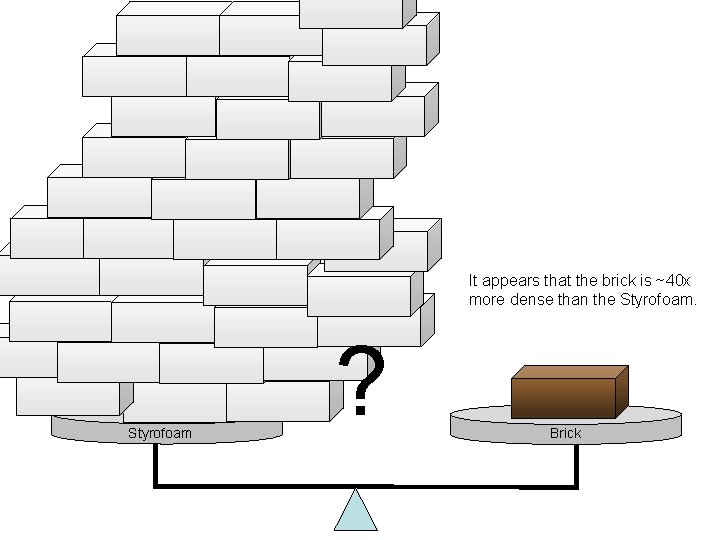
It appears that the brick is ~40 x more dense than the Styrofoam ? Brick
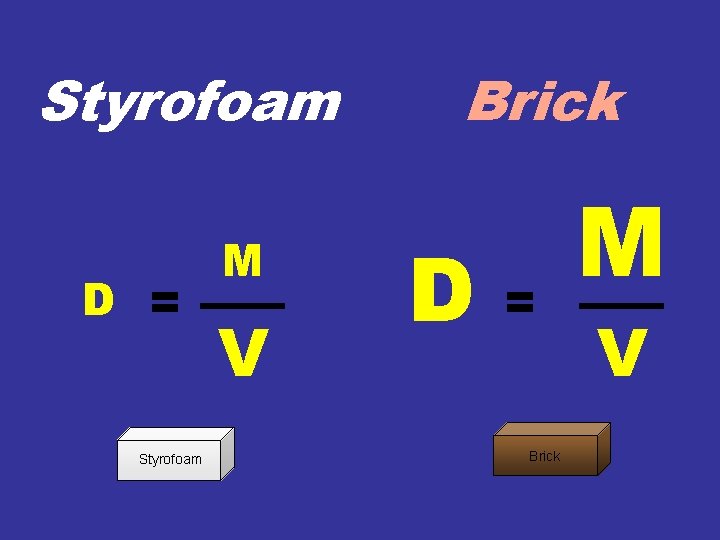
Styrofoam D = Styrofoam M V Brick D = Brick M V
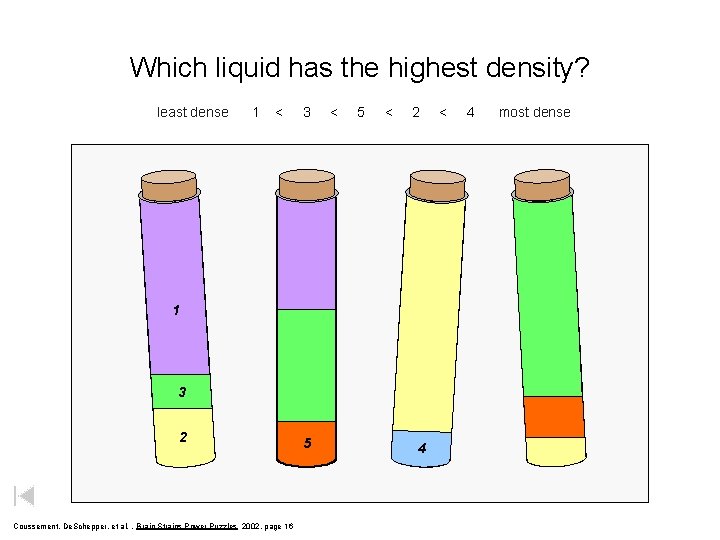
Which liquid has the highest density? least dense 1 < 3 < 5 < 2 1 3 2 Coussement, De. Schepper, et al. , Brain Strains Power Puzzles 2002, page 16 5 4 < 4 most dense
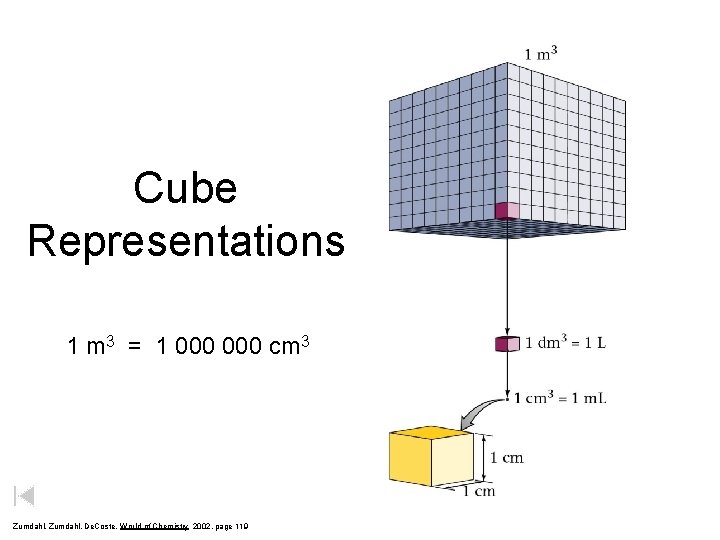
Cube Representations 1 m 3 = 1 000 cm 3 Zumdahl, De. Coste, World of Chemistry 2002, page 119
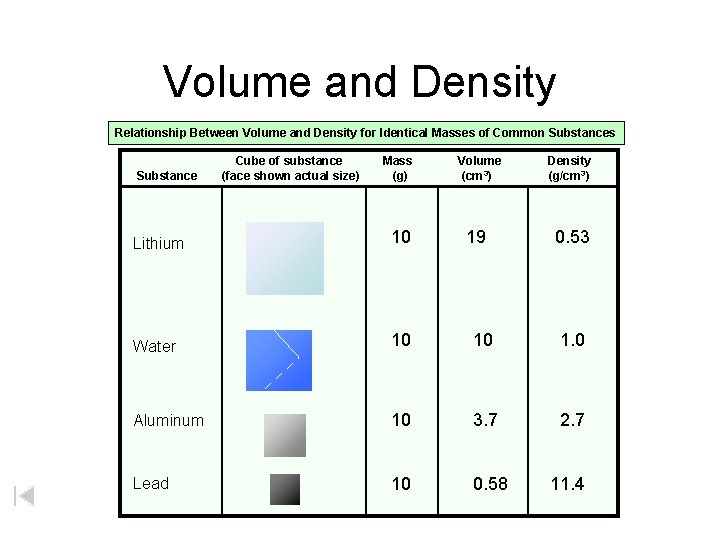
Volume and Density Relationship Between Volume and Density for Identical Masses of Common Substances Substance Cube of substance (face shown actual size) Mass (g) Volume (cm 3) 19 Density (g/cm 3) Lithium 10 0. 53 Water 10 10 1. 0 Aluminum 10 3. 7 2. 7 Lead 10 0. 58 11. 4
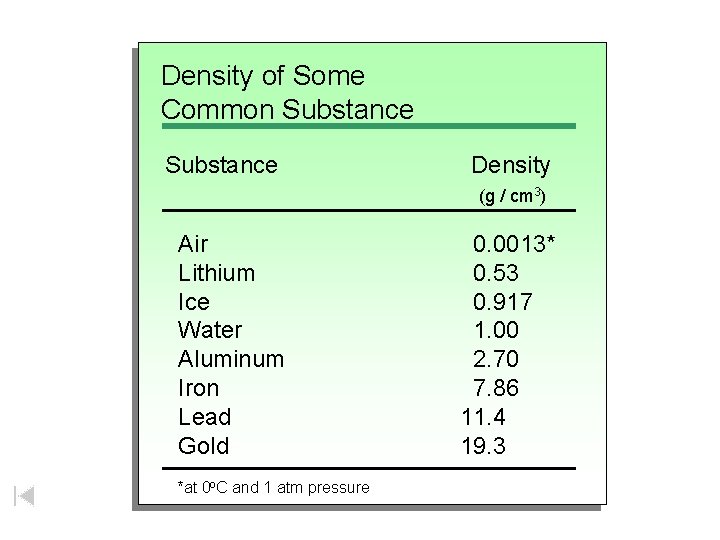
Density of Some Common Substances Substance Density (g / cm 3) Air Lithium Ice Water Aluminum Iron Lead Gold *at 0 o. C and 1 atm pressure 0. 0013* 0. 53 0. 917 1. 00 2. 70 7. 86 11. 4 19. 3
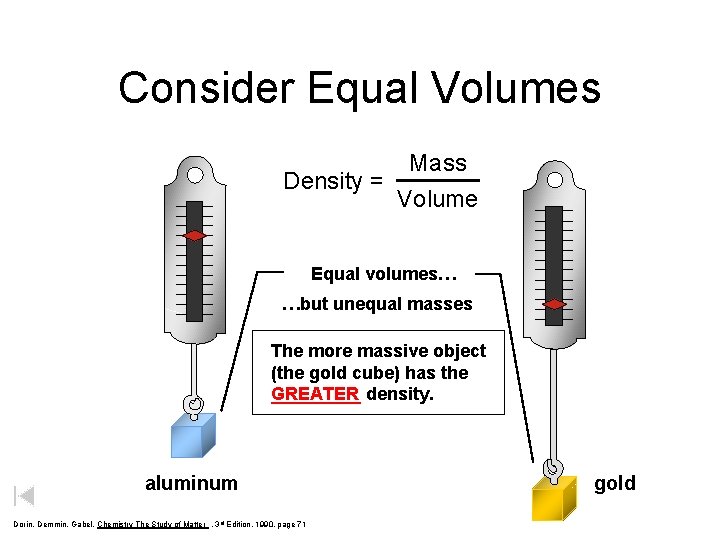
Consider Equal Volumes Mass Density = Volume Equal volumes… …but unequal masses The more massive object (the gold cube) has the GREATER density. _____ aluminum Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 71 gold
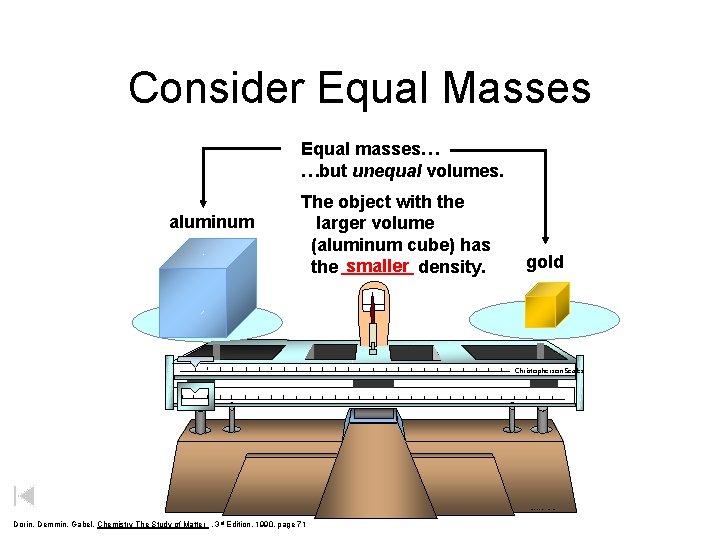
Consider Equal Masses Equal masses… …but unequal volumes. aluminum The object with the larger volume (aluminum cube) has the smaller density. gold Christopherson Scales Made in Normal, Illinois USA Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 71
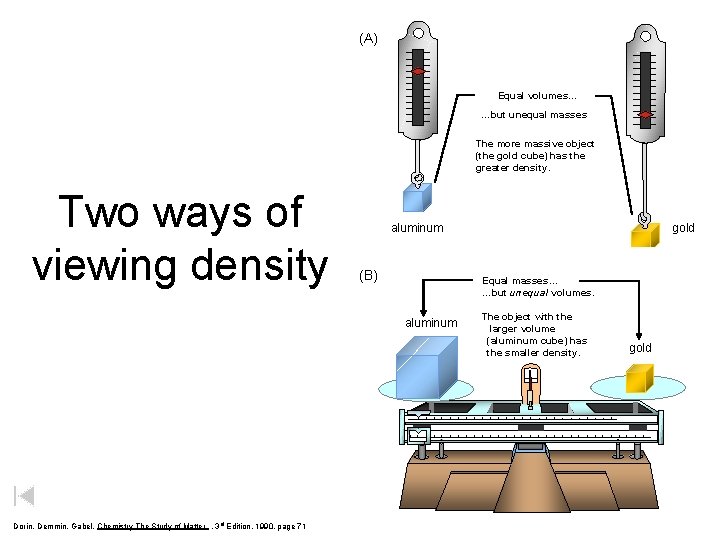
(A) Equal volumes… …but unequal masses The more massive object (the gold cube) has the greater density. Two ways of viewing density (B) Equal masses… …but unequal volumes. aluminum Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 71 gold aluminum The object with the larger volume (aluminum cube) has the smaller density. gold
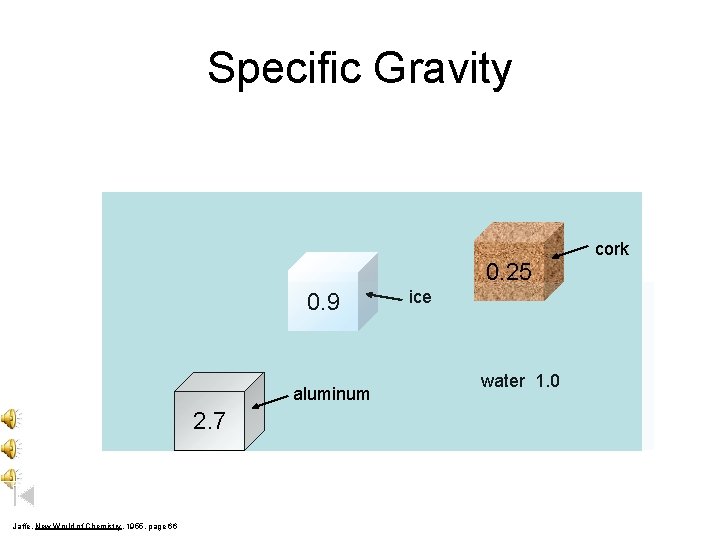
Specific Gravity 0. 25 0. 9 aluminum 2. 7 Jaffe, New World of Chemistry, 1955, page 66 ice water 1. 0 cork
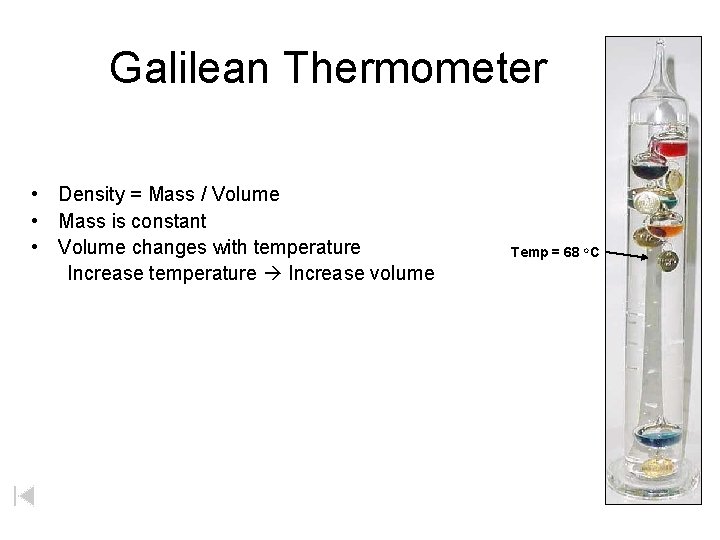
Galilean Thermometer • Density = Mass / Volume • Mass is constant • Volume changes with temperature Increase temperature Increase volume Temp = 68 o. C
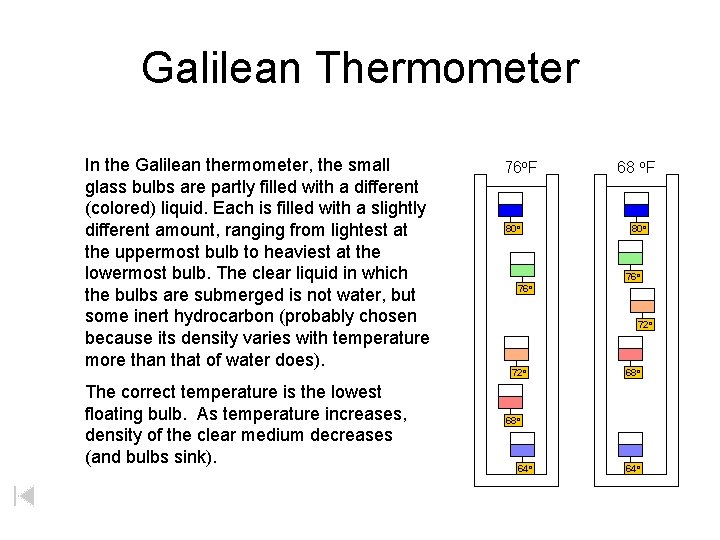
Galilean Thermometer In the Galilean thermometer, the small glass bulbs are partly filled with a different (colored) liquid. Each is filled with a slightly different amount, ranging from lightest at the uppermost bulb to heaviest at the lowermost bulb. The clear liquid in which the bulbs are submerged is not water, but some inert hydrocarbon (probably chosen because its density varies with temperature more than that of water does). The correct temperature is the lowest floating bulb. As temperature increases, density of the clear medium decreases (and bulbs sink). 76 o. F 80 o 68 o. F 80 o 76 o 72 o 68 o 64 o
- Slides: 23