Density Notes What is density l Density is
















- Slides: 18

Density Notes

What is density? l Density is a comparison of how much matter there is in a certain amount of space.

Which one is more dense? l Demonstration: People in a square l How about this: Circle which square is more dense?

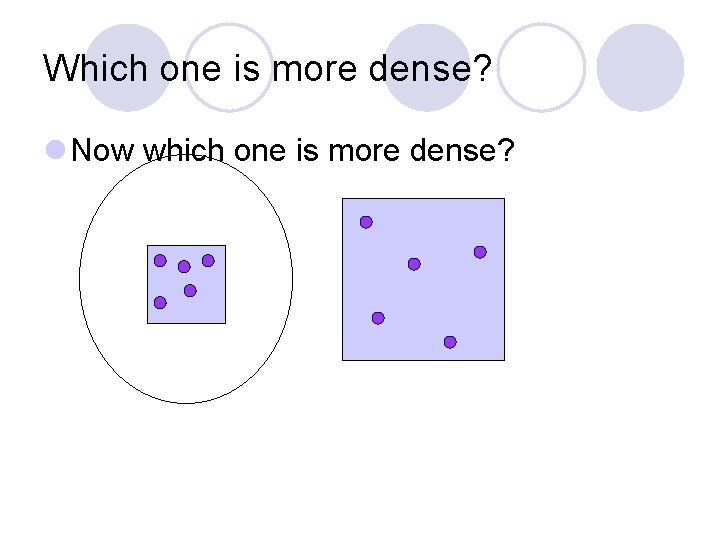
Which one is more dense? l Now which one is more dense?

What is density? l Density = mass OR volume mass ÷ volume. l Units for density: g OR g cm 3 m. L ALWAYS REMEMBER UNITS! l Why are these the units for density? Because grams is mass and cm 3 or m. L is volume!

Let’s try a density problem together l Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? l Frank also has an eraser. It has a mass of 3 g, and a volume of 1 cm 3. What is its density?

Work on these problems with your neighbor. l Jack has a rock. The rock has a mass of 6 g and a volume of 3 cm 3. What is the density of the rock? l Jill has a gel pen. The gel pen has a mass of 8 g and a volume of 2 cm 3. What is the density of the rock?

Now, try these on your own. l Al’Licia has a watch. It has a mass of 4 g and a volume of 2 cm 3. What is the density of the watch? l Mia has a wallet. It has a mass of 15 g and a volume of 5 cm 3. What is the density of the wallet?

Liquid Layers l If you pour together liquids that don’t mix and have different densities, they will form liquid layers. l The liquid with the highest density will be on the bottom. l The liquid with the lowest density will be on the top.

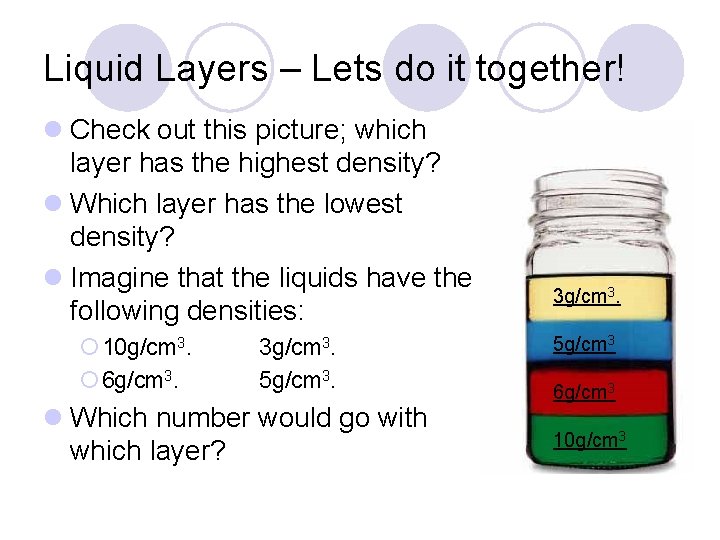
Liquid Layers – Lets do it together! l Check out this picture; which layer has the highest density? l Which layer has the lowest density? l Imagine that the liquids have the following densities: ¡ 10 g/cm 3. ¡ 6 g/cm 3. 3 g/cm 3. 5 g/cm 3. l Which number would go with which layer? 3 g/cm 3. 5 g/cm 3 6 g/cm 3 10 g/cm 3

Liquid Layers – Try with your neighbor l Which liquid has the highest density? l Which liquid has the lowest density? l Which liquid has the middle density?

Liquid Layers – Try on your own! l Imagine that the liquids on the right have the following densities: ¡ 15 g/cm 3 ¡ 3 g/cm 3 ¡ 7 g/cm 3 10 g/cm 3 9 g/cm 3 12 g/cm 3 l Match the colors to the correct densities. 3 g/cm 3 7 g/cm 3 9 g/cm 3 10 g/cm 3 12 g/cm 3 15 g/cm 3

Density of Water l The density of water is 1 g/m. L. l If an object has a density less than water, it will float in water l If an object has a density greater than water, it will sink in water

Will it sink or float? l A cedar wood chip has the density of 0. 8 g/m. L. Will it float or sink? ¡ 0. 8 g/m. L < 1. 0 g/m. L ¡Float! l A iron ball has the density of 7. 9 g/m. L. Will it float or sink? ¡ 7. 9 g/m. L < 1. 0 g/m. L ¡Sink!

Will it sink or float? l I have a toy boat that has the volume of 850 cm 3 and weighs 950 grams. Will it sink or float? l Sink! l How much do I need to decrease it’s weight to allow it to float? l At least 100 grams

Review l What is the formula for density? l What happens if you pour together liquids that have different densities?

Review l Will the liquid on the top have the highest or lowest density? l Will the liquid on the bottom have the highest or lowest density?

Super Scientist Question of the Day l Jake has a book, a ruler, and a balance. l How can Jake find the density of the book with the tools he has?