DENSITY NOTES Mrs DAnton UNIQUE PROPERTIES We need





- Slides: 8

DENSITY NOTES Mrs. D’Anton

UNIQUE PROPERTIES We need to be able to identify the materials we see every day so that we can use them correctly. We do this by observing physical and chemical properties and comparing them to what we know from personal experience. Physical properties include such things as boiling point, color, density, hardness, melting point, odor, taste, and even electrical conductivity. Chemical properties are usually a measure of how a material reacts or fails to react with other substances. Will it burn? Does it dissolve in water? Does it produce bubbles of gas dropped into acid? All of these things allow us to tell the difference between water and alcohol, for example, and many other substances with the use of only one or two of our senses. List at least five of the physical and chemical properties of water and alcohol. What simple test could you do to determine which liquid was which? 3

OBJECTIVES - Explain the difference between intensive and extensive physical properties - Review density concepts & relationships - Practice manipulating and solving algebraic equations involving density

TERMS Mass: The amount of a substance Volume: The space a substance takes up Density: The ratio of mass to volume of a substance, often expressed in grams per liter or grams per cubic centimeter Physical Property: A characteristic of a substance that does not involve chemical change Extensive Property: A physical property that does depend on the amount of a substance Intensive Property: A physical property that does not depend on the amount of a substance Slope: The ratio of rise to run. Dependent Variable: A variable that is changed as a result of the experiment. Independent Variable: A variable that is manipulated or varied in an experiment. Conversion Factor: Ratio that is derived from the equality of two different units and can be used to convert from one unit to another

WHAT IS DENSITY? Density is an example of an intensive physical property. Density of a substance is the same for all samples of that substance. 50 g of aluminum foil has the same density as 1 g of aluminum foil Milk in a half gallon container has the same density as milk in a gallon container

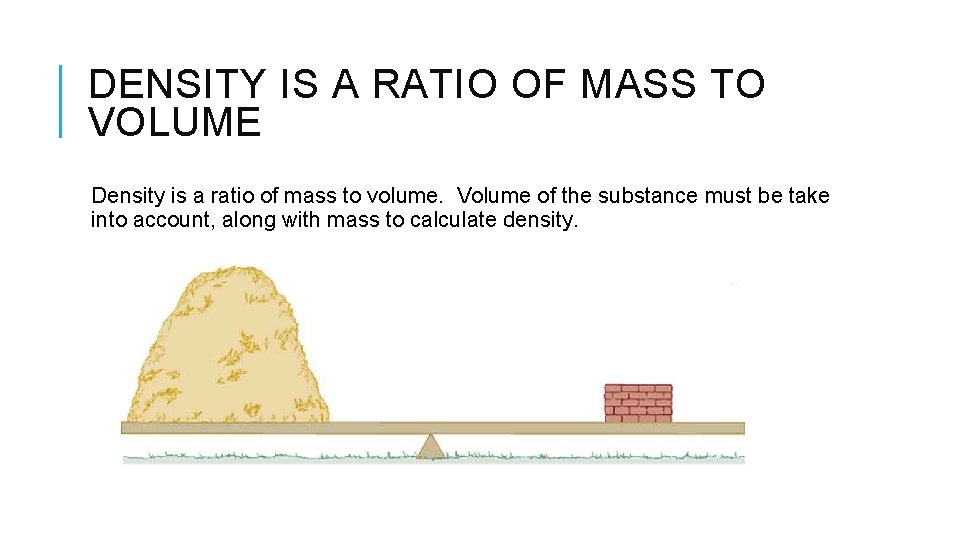
DENSITY IS A RATIO OF MASS TO VOLUME Density is a ratio of mass to volume. Volume of the substance must be take into account, along with mass to calculate density.

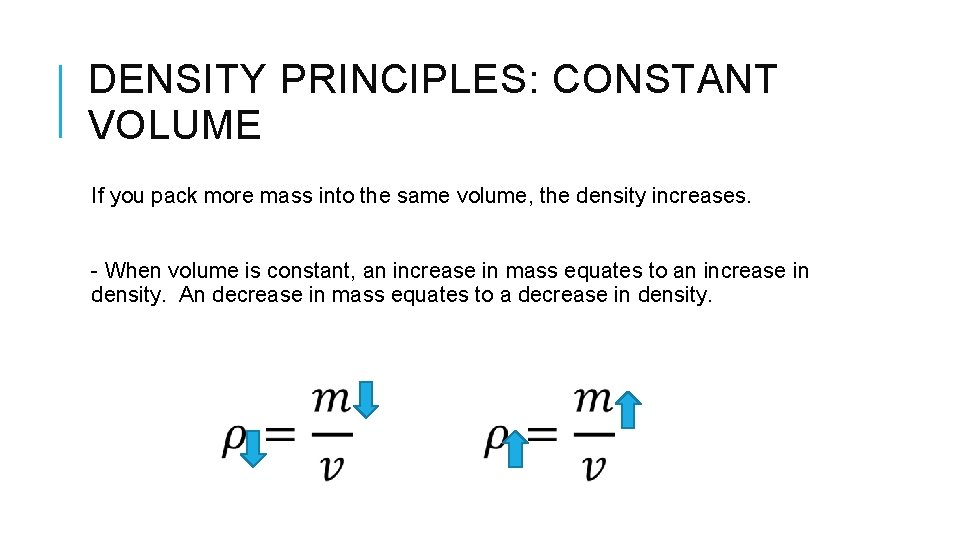
DENSITY PRINCIPLES: CONSTANT VOLUME If you pack more mass into the same volume, the density increases. - When volume is constant, an increase in mass equates to an increase in density. An decrease in mass equates to a decrease in density.

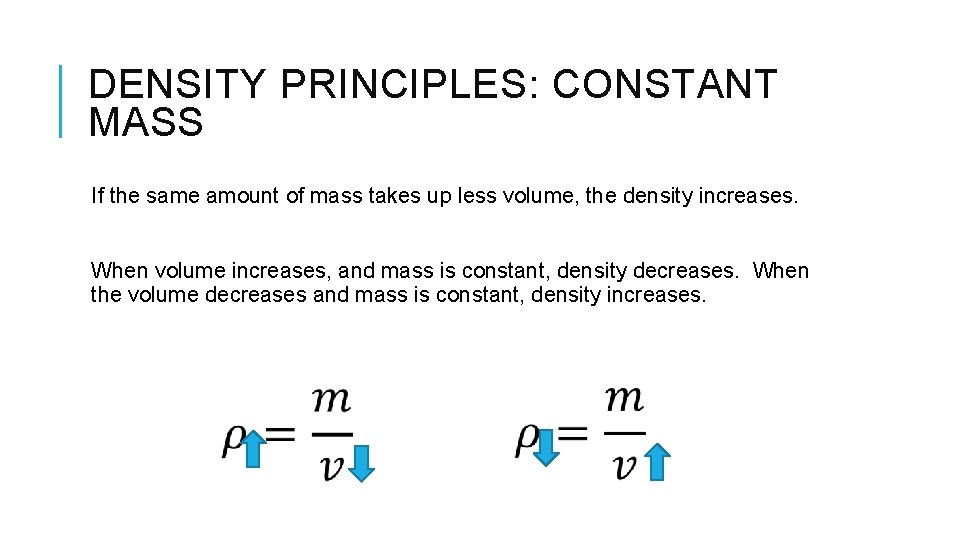
DENSITY PRINCIPLES: CONSTANT MASS If the same amount of mass takes up less volume, the density increases. When volume increases, and mass is constant, density decreases. When the volume decreases and mass is constant, density increases.