Density Notes EQ What is density A Mass





- Slides: 19

Density Notes EQ: What is density?

A. Mass 1. Measured in grams (g) 2. Mass vs weight Depends on the matter in the attraction of gravity object


B. Volume 1. Measured in cm 3, m. L, L, … 2. Amount of space taken up by an object

Ways to Find Volume 1. Measuring distance and use an equation

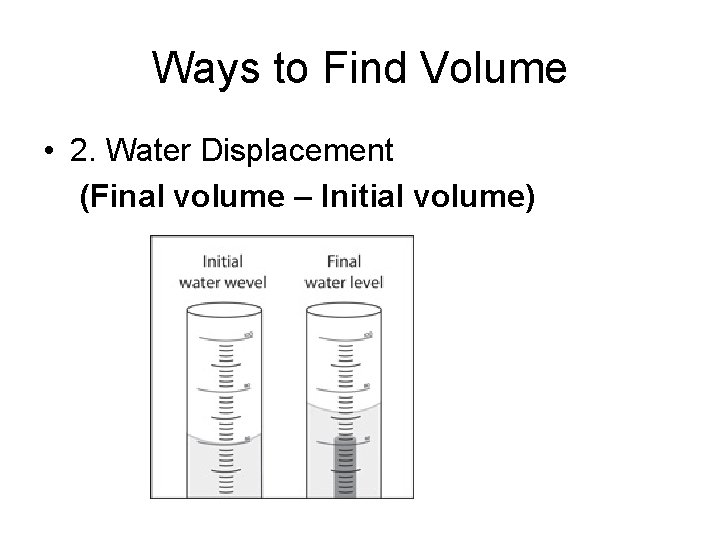
Ways to Find Volume • 2. Water Displacement (Final volume – Initial volume)

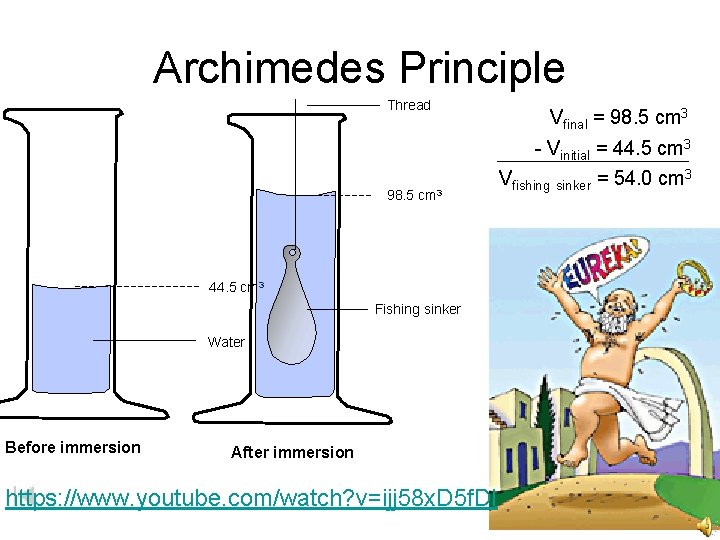
Archimedes Principle Thread 98. 5 cm 3 44. 5 cm 3 Fishing sinker Water Before immersion After immersion https: //www. youtube. com/watch? v=ijj 58 x. D 5 f. DI Vfinal = 98. 5 cm 3 - Vinitial = 44. 5 cm 3 Vfishing sinker = 54. 0 cm 3

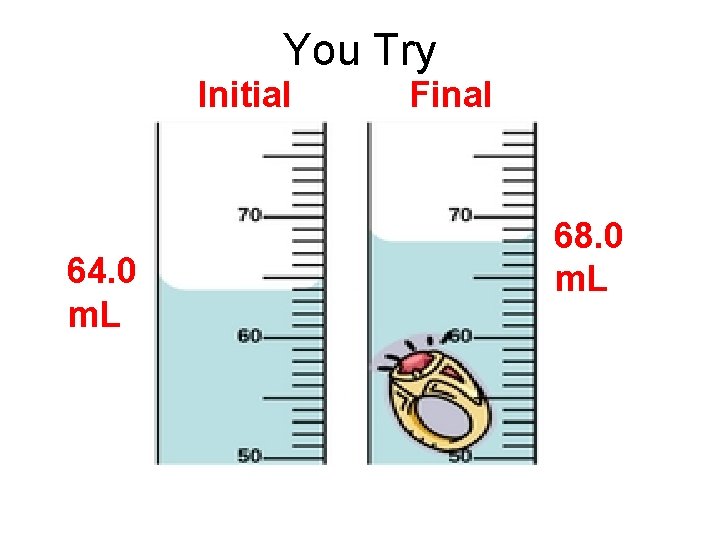
You Try Initial 64. 0 m. L Final 68. 0 m. L

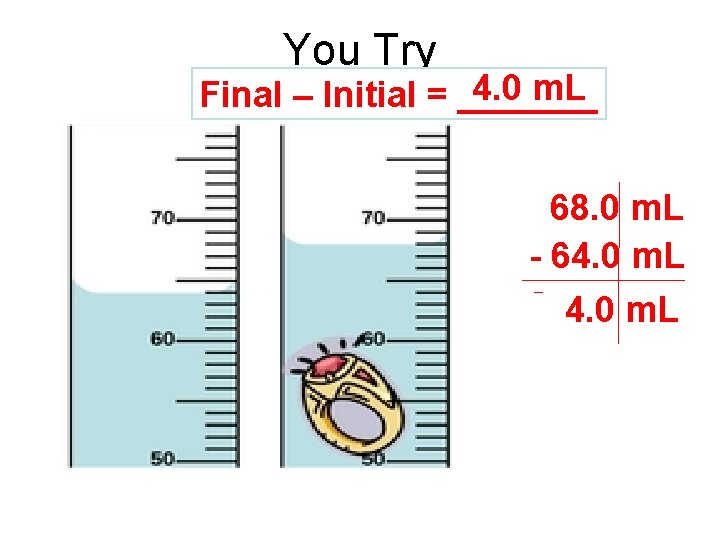
You Try 4. 0 m. L Final – Initial = _______ 68. 0 m. L - 64. 0 m. L

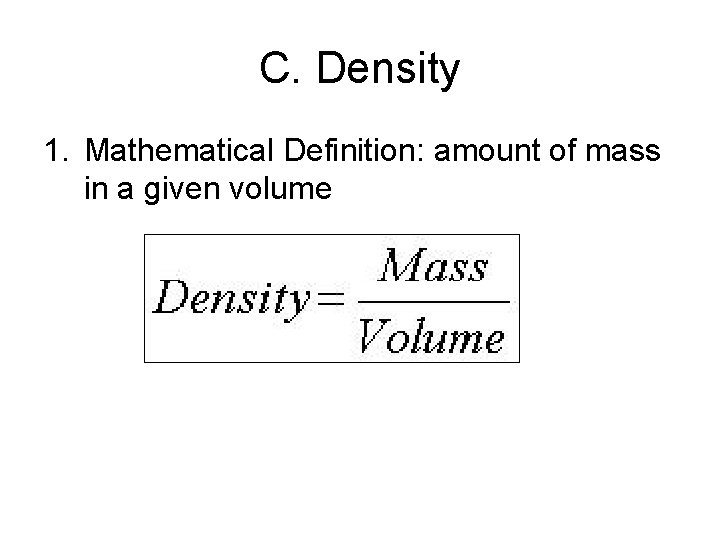
C. Density 1. Mathematical Definition: amount of mass in a given volume

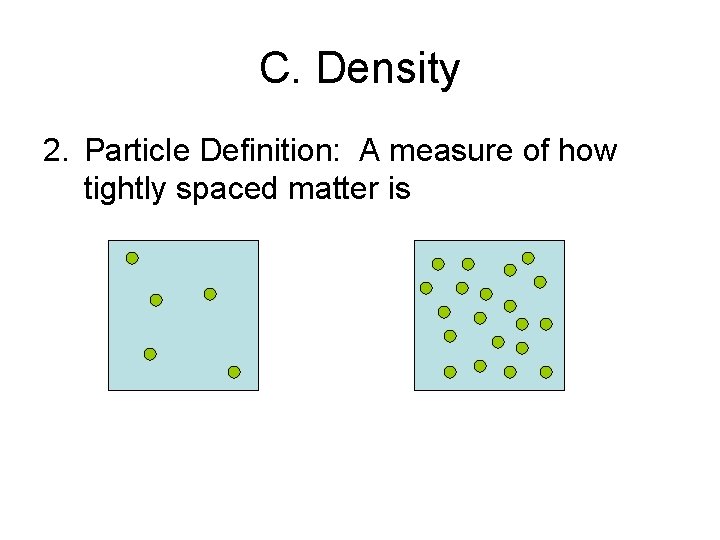
C. Density 2. Particle Definition: A measure of how tightly spaced matter is

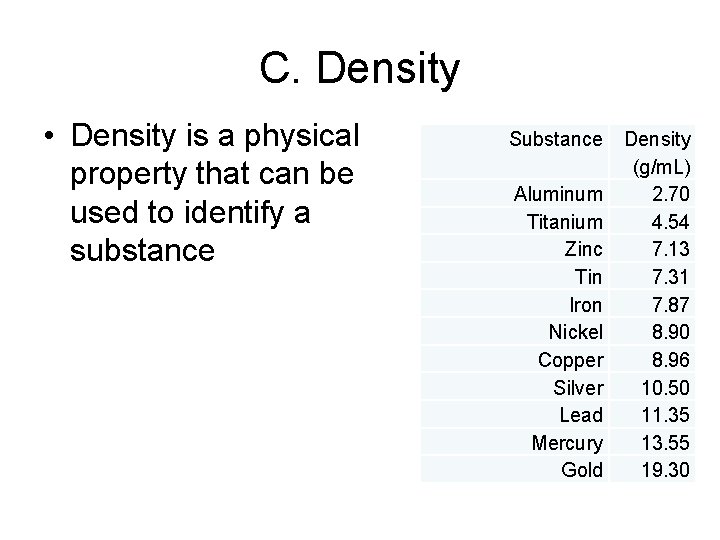
C. Density • Density is a physical property that can be used to identify a substance Substance Aluminum Titanium Zinc Tin Iron Nickel Copper Silver Lead Mercury Gold Density (g/m. L) 2. 70 4. 54 7. 13 7. 31 7. 87 8. 90 8. 96 10. 50 11. 35 13. 55 19. 30

D. Density Practice 1. A piece of metal with a mass of 1. 58 grams was placed into a graduated cylinder filled with 25. 65 ml of water and the water level rose to 25. 87 ml.

Problem solving in Chemistry GUESS G- Givens U- Unknowns E- Equation S- Substitute S – Solve Don’t forget to round your answer to the correct number of significant Figures

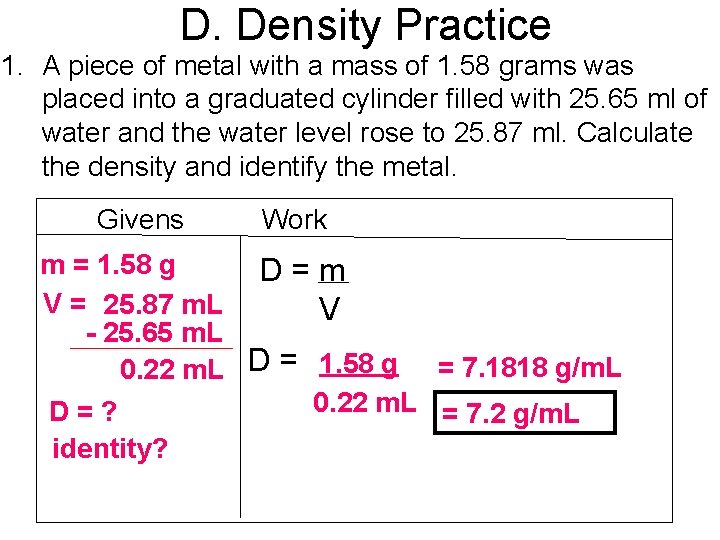
D. Density Practice 1. A piece of metal with a mass of 1. 58 grams was placed into a graduated cylinder filled with 25. 65 ml of water and the water level rose to 25. 87 ml. Calculate the density and identify the metal. Givens Work m = 1. 58 g D=m V = 25. 87 m. L V - 25. 65 m. L 0. 22 m. L D = 1. 58 g = 7. 1818 g/m. L 0. 22 m. L = 7. 2 g/m. L D=? identity?

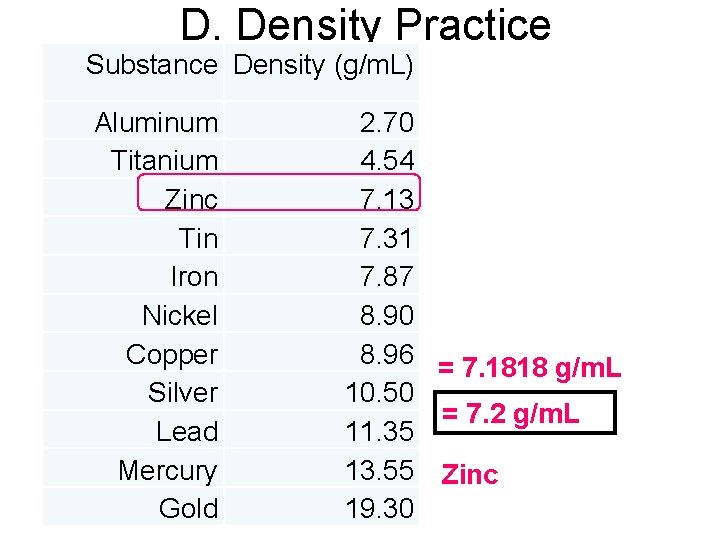
D. Density Practice Substance Density (g/m. L) Aluminum 2. 70 Titanium 4. 54 Zinc 7. 13 Givens Work Tin 7. 31 m = 1. 58 g. Iron D = m 7. 87 V = 25. 87 m. L V 8. 90 Nickel - 25. 65 m. L Copper D = 1. 588. 96 g = 7. 1818 g/m. L 0. 22 m. L Silver 10. 50 0. 22 m. L = 7. 2 g/m. L D=? Lead 11. 35 identity? Mercury 13. 55 Zinc Gold 19. 30

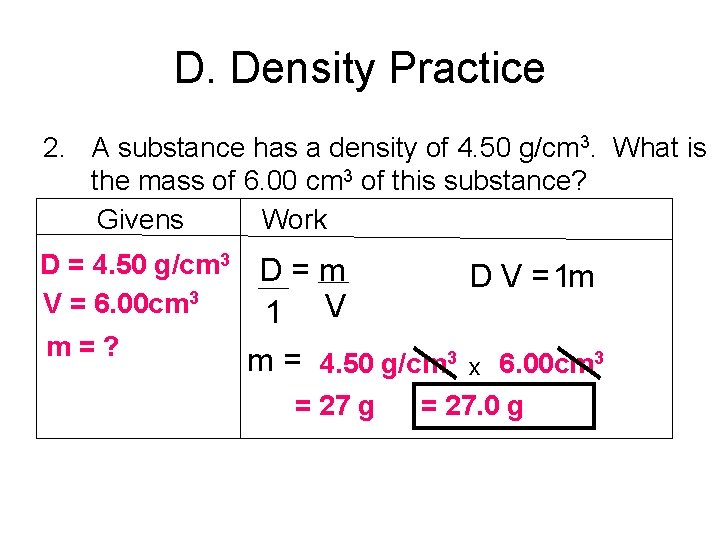
D. Density Practice 2. A substance has a density of 4. 50 g/cm 3. What is the mass of 6. 00 cm 3 of this substance? Givens Work D = 4. 50 g/cm 3 V = 6. 00 cm 3 m=? D=m D V = 1 m 1 V m = 4. 50 g/cm 3 x 6. 00 cm 3 = 27 g = 27. 0 g

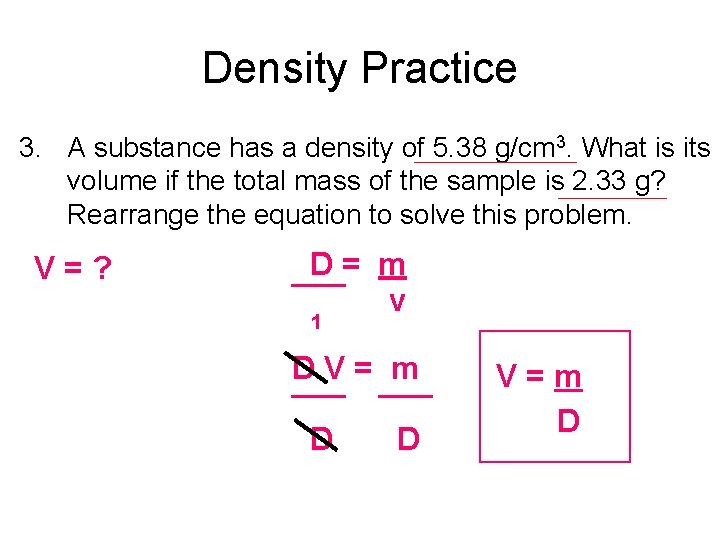
Density Practice 3. A substance has a density of 5. 38 g/cm 3. What is its volume if the total mass of the sample is 2. 33 g? Rearrange the equation to solve this problem. V=? D= m ___ v 1 D V = m ___ D D V=m D

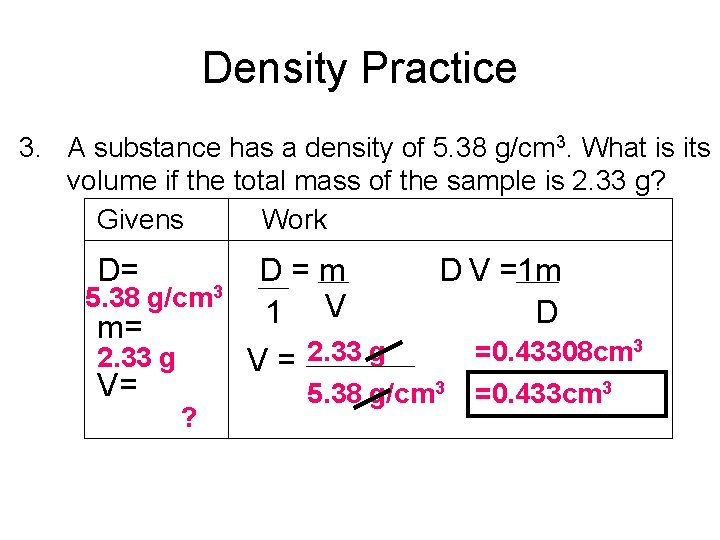
Density Practice 3. A substance has a density of 5. 38 g/cm 3. What is its volume if the total mass of the sample is 2. 33 g? Givens Work D= 5. 38 g/cm 3 m= 2. 33 g V= ? D=m 1 V V = 2. 33 g D V =1 m D 5. 38 g/cm 3 =0. 43308 cm 3 =0. 433 cm 3