DENSITY MASS WEIGHT VOLUME UNIT SUMMARY BY DIRECTIONS

DENSITY MASS, WEIGHT & VOLUME UNIT SUMMARY BY: ___________

DIRECTIONS: • Download this PPT to use it as a template for your assignment. • There are prompts on each slide with instructions. • Delete the instructions from the slide after you have completed the slide.
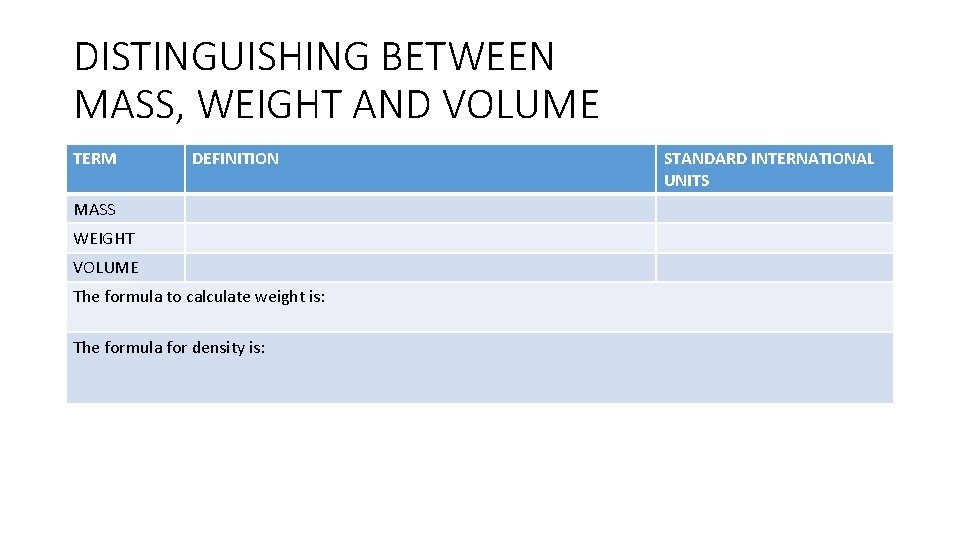
DISTINGUISHING BETWEEN MASS, WEIGHT AND VOLUME TERM DEFINITION MASS WEIGHT VOLUME The formula to calculate weight is: The formula for density is: STANDARD INTERNATIONAL UNITS
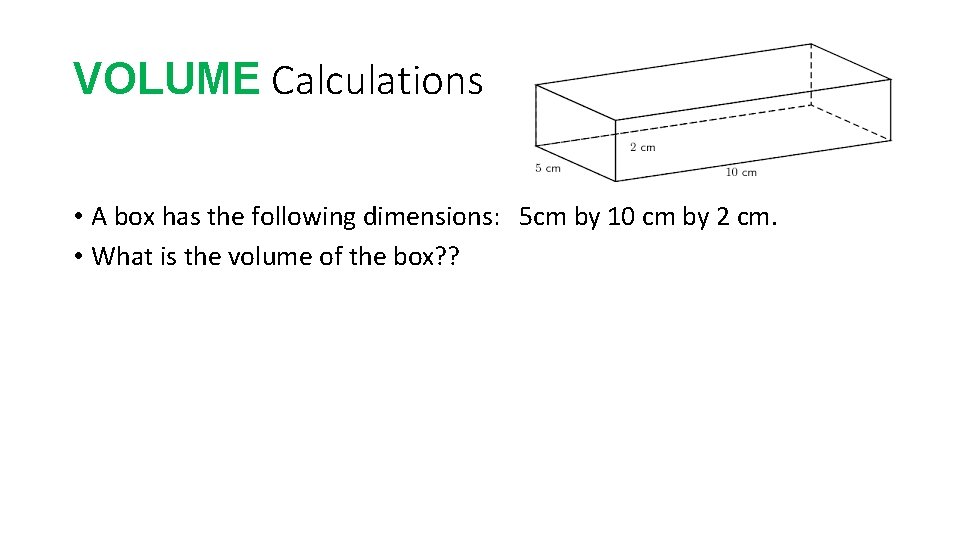
VOLUME Calculations • A box has the following dimensions: 5 cm by 10 cm by 2 cm. • What is the volume of the box? ?

Relationship between mass and Weight • Formula: • Find the weight of 120 kg object on the earth. • Will it weight more or less on the moon? Why? m = 120 kg

SAME MASS, DIFFERENT VOLUMES 1. EXPLAIN HOW TW O OBJECTS OF THE SAME MASS CAN HA VE DIFFERENT VOLUMES. 2. INCLUDE AN IMA GE SHOW THIS IDEA HINT: IT TAKES A H UGE PILE OF FEATHERS TO HAVE THE SAME MASS AS A PILE OF ROCKS

SAME VOLUME, DIFFERENT MASSES 1. EXPLAIN HOW TW O OBJECTS OF THE SAME VOLUME CAN HAVE DIFFERENT MASS. 2. INCLUDE AN IMA GE SHOW THIS IDEA HINT: IT TAKES A H UGE PILE OF FEATHERS TO HAVE THE SAME MASS AS A PILE OF ROCKS

DENSITY DEFINITION: INC L ILLU UDE A N STR ATE IMAG THE E TO H DEF INIT ELP ION.

Density of liquid water • What is the density of liquid water at 4 o C? Look in Physi your Boo cs on k page of help you w 1 ith th 7 to is on e • If an iceberg’s mass is 300, 000 grams, find its Volume. • Hint: The density of ice is 0. 917 g/(cm 3) .

Density Calculations • If a ball’s density is 2. 3 g/m. L, what is its volume if its mass is 6 g? • Will it float or sink in liquid water?

Density Calculations • If a ball’s density is 2. 3 g/m. L, what is its mass if its volume is 8 m. L? • Will it float or sink in liquid water?

Density Calculations • Find the density of a box with a volume of 45 cm 3 and a mass of 4. 5 kg. • Will it float or sink in liquid water?

Density Calculations • A 6 kg box has the following dimensions: 5 cm by 1 cm by 2 cm. • What is the density of the box? ? • Will it float or sink in liquid water?

You add water and alcohol to a graduated cylinder(GC). The density of alcohol is 0. 91 g/m. L. You drop a ball in to the GC with the liquid. The ball is more dense than alcohol and less dense than water. Sketch the liquids and the ball in the GC. Explain the behavior of the ball.
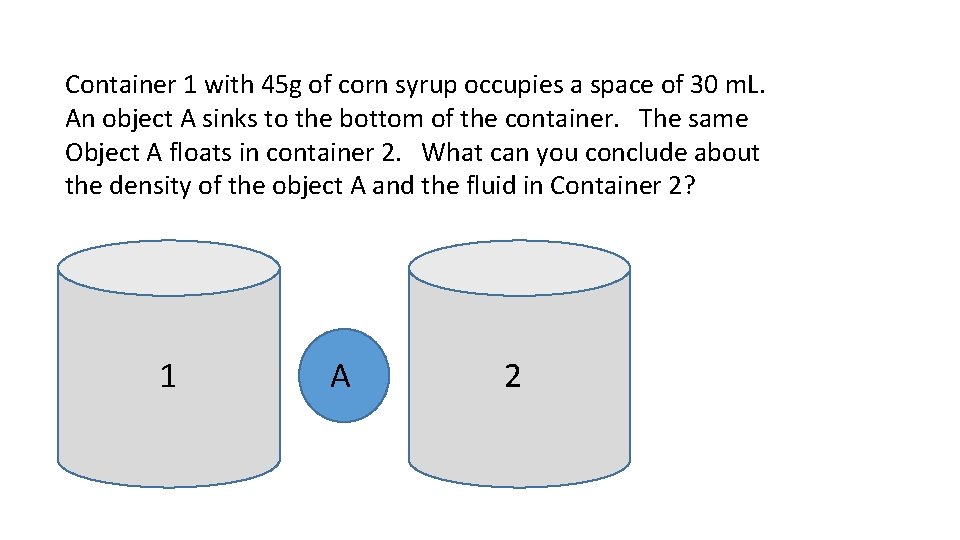
Container 1 with 45 g of corn syrup occupies a space of 30 m. L. An object A sinks to the bottom of the container. The same Object A floats in container 2. What can you conclude about the density of the object A and the fluid in Container 2? 1 A 2
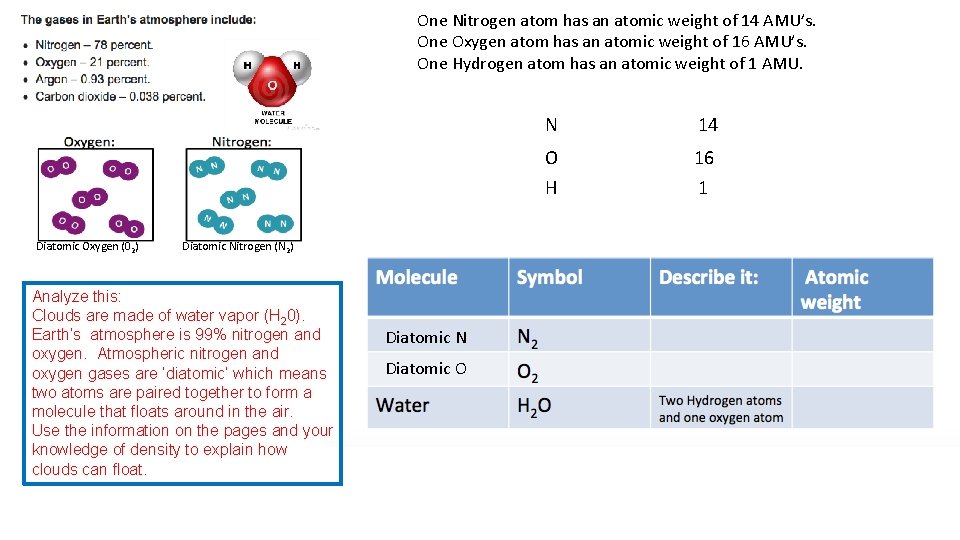
One Nitrogen atom has an atomic weight of 14 AMU’s. One Oxygen atom has an atomic weight of 16 AMU’s. One Hydrogen atom has an atomic weight of 1 AMU. Diatomic Oxygen (02) Diatomic Nitrogen (N 2) Analyze this: Clouds are made of water vapor (H 20). Earth’s atmosphere is 99% nitrogen and oxygen. Atmospheric nitrogen and oxygen gases are ‘diatomic’ which means two atoms are paired together to form a molecule that floats around in the air. Use the information on the pages and your knowledge of density to explain how clouds can float. Diatomic N Diatomic O N 14 O 16 H 1



- Slides: 19