Density Lab t Title Density Lab Aim purpose

Density Lab t

Title: Density Lab

Aim (purpose) To classify various materials in increasing order of density.

Materials Triple beam balance 2 regular samples, 2 irregular samples 100 m. L graduated cylinder Ruler

Procedure: Water 1. Find the mass of the empty graduated cylinder. Record this mass. 2. Add a fixed volume of water. Record this volume. 3. Find the mass of the graduated cylinder and water. Subtract the two masses to obtain the mass of water. 4. Calculate density using: mass (g)/volume (m. L)
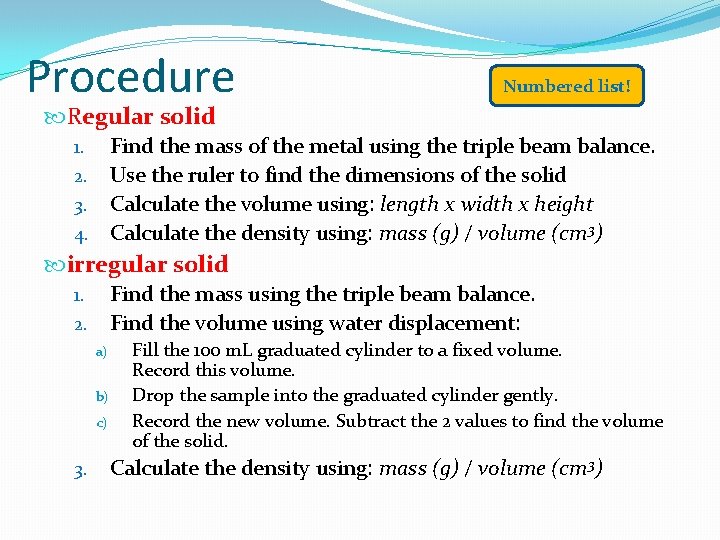
Procedure Numbered list! Regular solid Find the mass of the metal using the triple beam balance. 2. Use the ruler to find the dimensions of the solid 3. Calculate the volume using: length x width x height 4. Calculate the density using: mass (g) / volume (cm 3) 1. irregular solid Find the mass using the triple beam balance. 2. Find the volume using water displacement: 1. a) b) c) 3. Fill the 100 m. L graduated cylinder to a fixed volume. Record this volume. Drop the sample into the graduated cylinder gently. Record the new volume. Subtract the 2 values to find the volume of the solid. Calculate the density using: mass (g) / volume (cm 3)

Results (data) Do not forget your UNITS!!! Sample A: regular solid DIMENSIONS Mass Length Width Height Sample B: irregular solid VOLUME Mass Initial volume Final Volume Object
Analysis Sample A: regular solid Sample B: irregular solid CALCULATIONS -Formulas -Units
Conclusion The density of the substances was determined and placed in increasing order or density. Using the values for mass and volume recorded in the data table of the Results section and applying the formula for density (ρ = m/v), the density for the samples were calculated and compared. Sample _____’s density is ____, while sample _____ has a density of _____, as shown in the Analysis section.

Important reminders Return all materials as they were found to their proper locations. Clean station Write in full sentences (except materials and procedure)
- Slides: 10