Density Functional Theory DFT DFT is an alternative

![Properties of the electron density Function: y=f(x) ρ= ρ(x, y, z) Functional: y=F[f(x)] E=F[ρ(x, Properties of the electron density Function: y=f(x) ρ= ρ(x, y, z) Functional: y=F[f(x)] E=F[ρ(x,](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-3.jpg)

![T[ρ] – kinetic energy of the system Kohn and Sham proposed to calculate the T[ρ] – kinetic energy of the system Kohn and Sham proposed to calculate the](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-8.jpg)
![Kohn-Sham Equations: Minimize E[ρ] with the conditions: with: Kohn-Sham Equations: Minimize E[ρ] with the conditions: with:](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-9.jpg)
![Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-11.jpg)



- Slides: 17

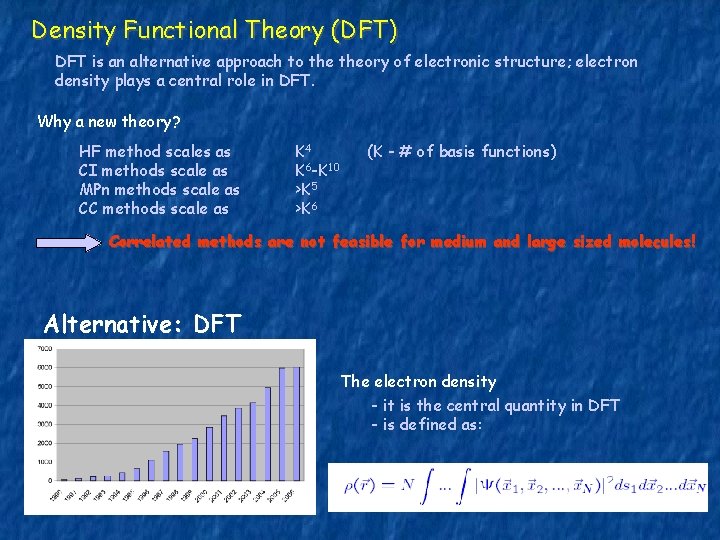
Density Functional Theory (DFT) DFT is an alternative approach to theory of electronic structure; electron density plays a central role in DFT. Why a new theory? HF method scales as CI methods scale as MPn methods scale as CC methods scale as K 4 K 6 -K 10 >K 5 >K 6 (K - # of basis functions) Correlated methods are not feasible for medium and large sized molecules! Alternative: DFT The electron density - it is the central quantity in DFT - is defined as:

The electron density
![Properties of the electron density Function yfx ρ ρx y z Functional yFfx EFρx Properties of the electron density Function: y=f(x) ρ= ρ(x, y, z) Functional: y=F[f(x)] E=F[ρ(x,](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-3.jpg)
Properties of the electron density Function: y=f(x) ρ= ρ(x, y, z) Functional: y=F[f(x)] E=F[ρ(x, y, z)]

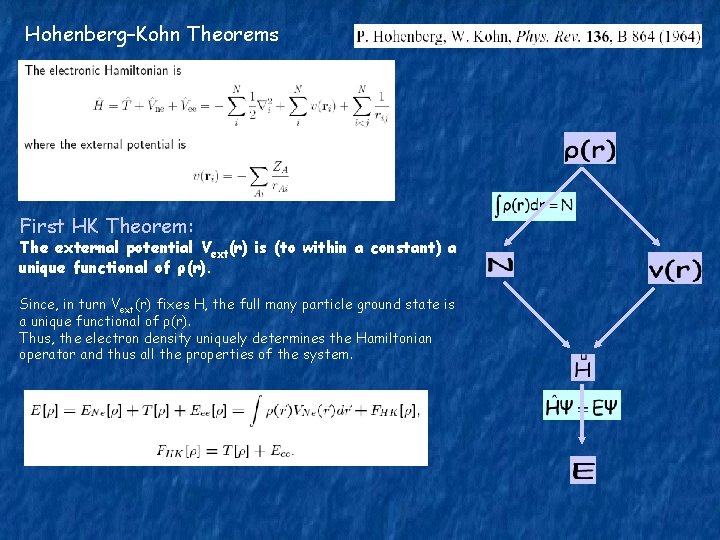
Hohenberg–Kohn Theorems First HK Theorem: The external potential Vext(r) is (to within a constant) a unique functional of ρ(r). Since, in turn Vext(r) fixes H, the full many particle ground state is a unique functional of ρ(r). Thus, the electron density uniquely determines the Hamiltonian operator and thus all the properties of the system.

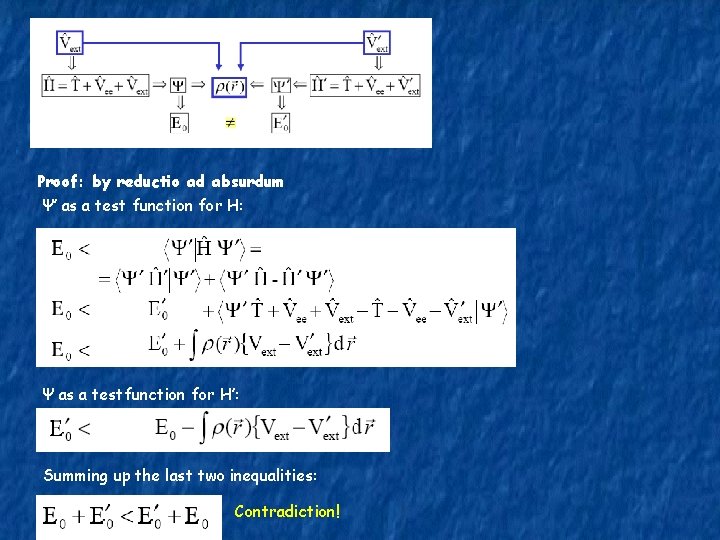
Proof: by reductio ad absurdum Ψ’ as a test function for H: Ψ as a testfunction for H’: Summing up the last two inequalities: Contradiction!

Variational Principle in DFT Second HK Theorem The functional that delivers the ground state energy of the system, delivers the lowest energy if and only if the input density is the true ground state density. - variational principle For any trial density ρ(r), which satisfies the necessary boundary conditions such as: ρ(r) 0 and which is associated with some external potential V ext, the energy obtained from the functional of FHK represents an upper bound to the true ground state energy E 0.

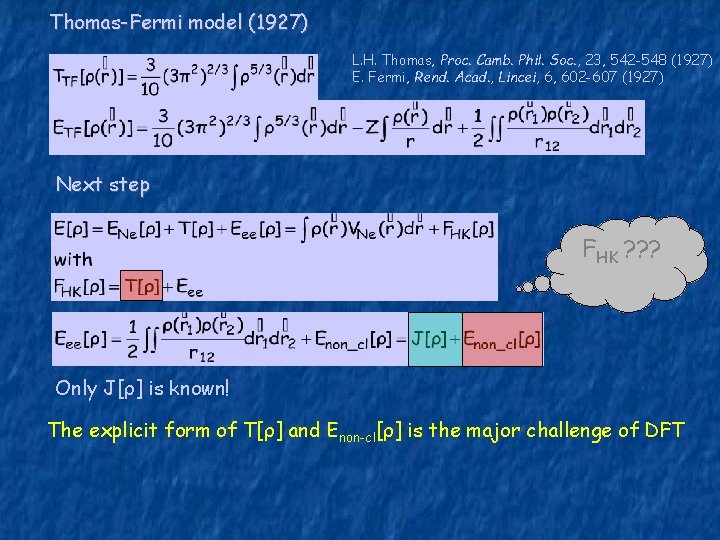
Thomas-Fermi model (1927) L. H. Thomas, Proc. Camb. Phil. Soc. , 23, 542 -548 (1927) E. Fermi, Rend. Acad. , Lincei, 6, 602 -607 (1927) Next step FHK ? ? ? Only J[ρ] is known! The explicit form of T[ρ] and Enon-cl[ρ] is the major challenge of DFT
![Tρ kinetic energy of the system Kohn and Sham proposed to calculate the T[ρ] – kinetic energy of the system Kohn and Sham proposed to calculate the](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-8.jpg)
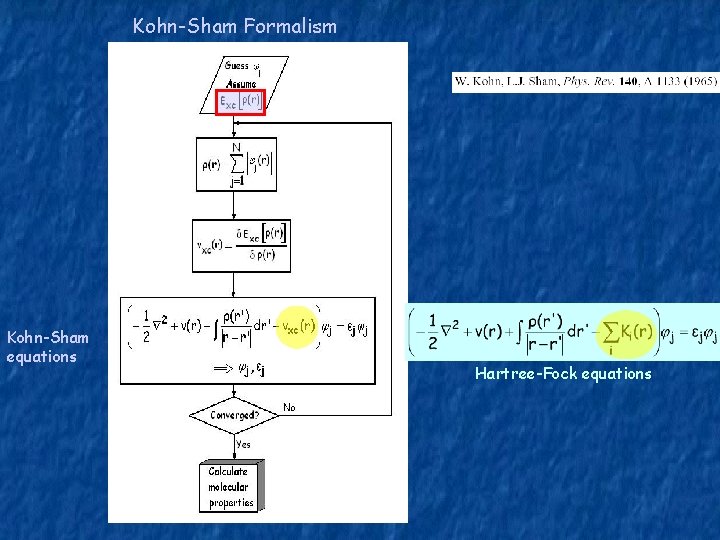
T[ρ] – kinetic energy of the system Kohn and Sham proposed to calculate the exact kinetic energy of a non-interacting system with the same density as for the real interacting system. TKS Ψi – kinetic energy of a fictitious non-interacting system of the same density ρ(r) - are the orbitals for the non-interacting system (KS orbitals) T=TKS+(T-TKS) Exc[ρ] includes everything which is unknown: - exchange energy - correlation energy - correction of kinetic energy (T-TKS)
![KohnSham Equations Minimize Eρ with the conditions with Kohn-Sham Equations: Minimize E[ρ] with the conditions: with:](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-9.jpg)
Kohn-Sham Equations: Minimize E[ρ] with the conditions: with:

Kohn-Sham Formalism Kohn-Sham equations Hartree-Fock equations
![Excρ Local Density Approximation LDA εxc only depends on the density Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density](https://slidetodoc.com/presentation_image_h/ec80504c24d528d05f1d735f0f4bc353/image-11.jpg)
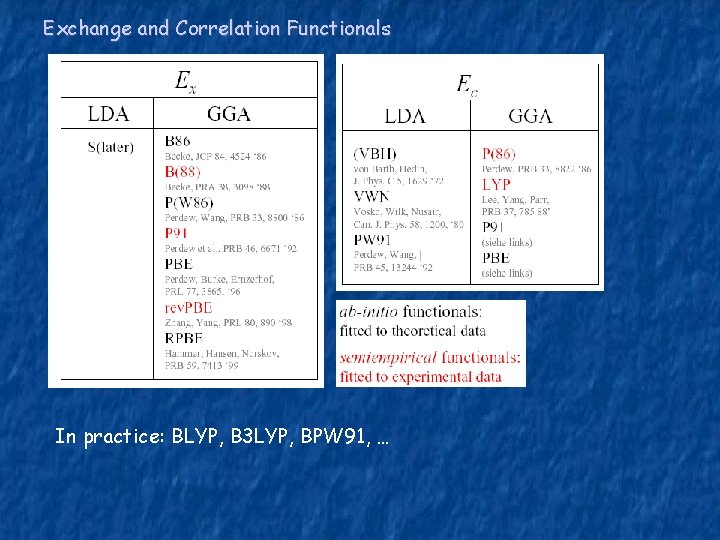
Exc[ρ] = ? ? Local Density Approximation (LDA) εxc only depends on the density at r For the correlation part: Monte-Carlo simulations – Ceperly and Alder Good for solids Generalized Gradient Approximation (GGA) εxc depends on the density and its gradient at r (1) Adjust εxc such that it satises all (or most) known properties of the exchangecorrelation hole and energy. (2) PW 91, PBE… (2) Fit εxc to a large data-set own exactly known binding energies of atoms and molecules. BLYP, OLYP, HCTH…


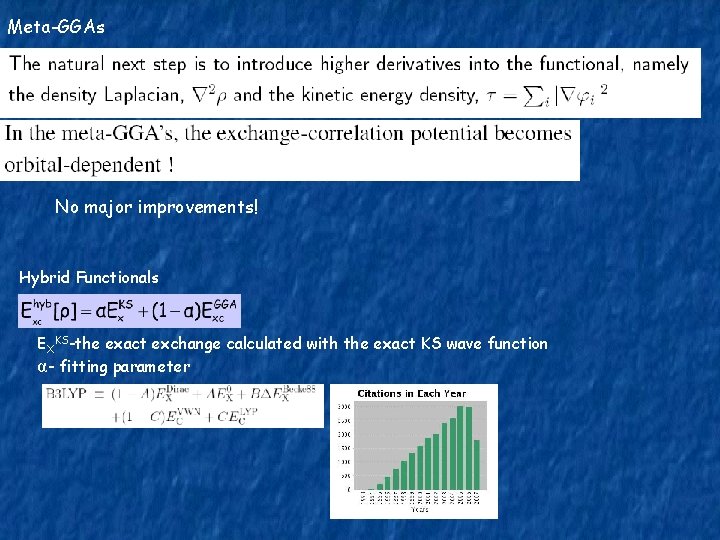
Meta-GGAs No major improvements! Hybrid Functionals EXKS-the exact exchange calculated with the exact KS wave function α- fitting parameter

Exchange and Correlation Functionals In practice: BLYP, B 3 LYP, BPW 91, …


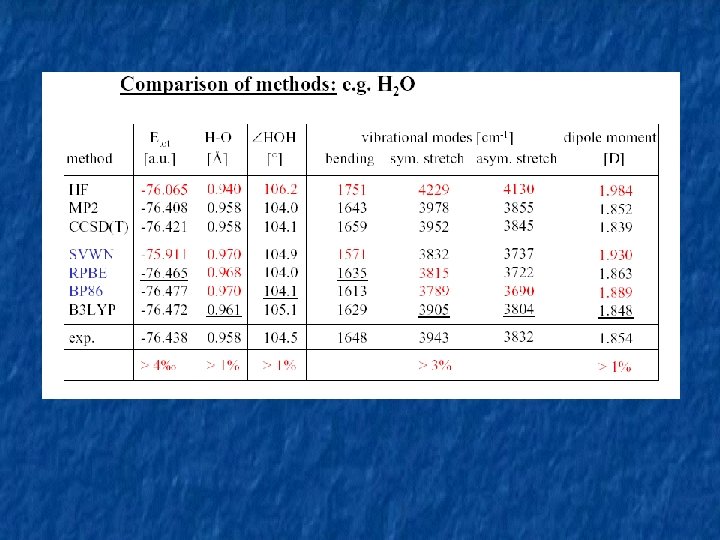
Different functionals for different properties Atomization energies: Ionization energy: - B 3 LYP – the best! Electron afinities: Vibrational frequencies: - (BLYP), B 3 LYP, … MO 5 -2 X - bond dissociation energies, stacking and hydrogen-bonding interactions in nucleobase pairs

Kohn-Sham orbitals