Density Experiment 3 Density Density of a rubber

Density Experiment # 3
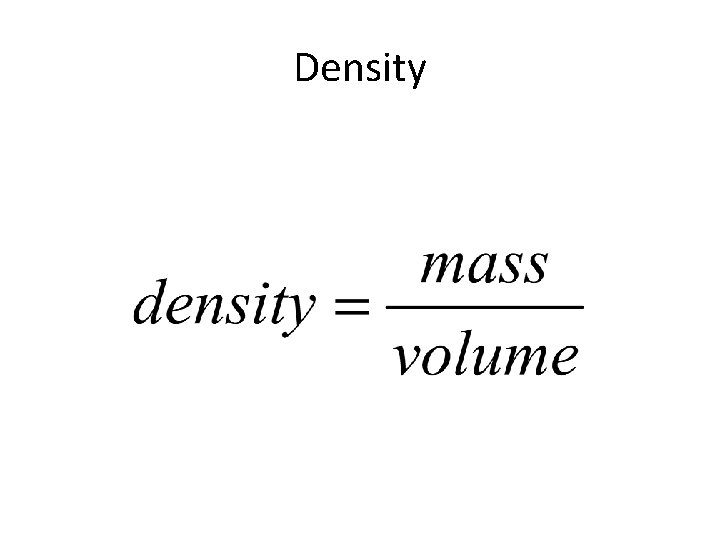
Density

Density of a rubber stopper • Determine density be displacement of water Add rubber stopper Fill graduated cylinder with a known volume of water. Determine total volume of rubber stopper and water.
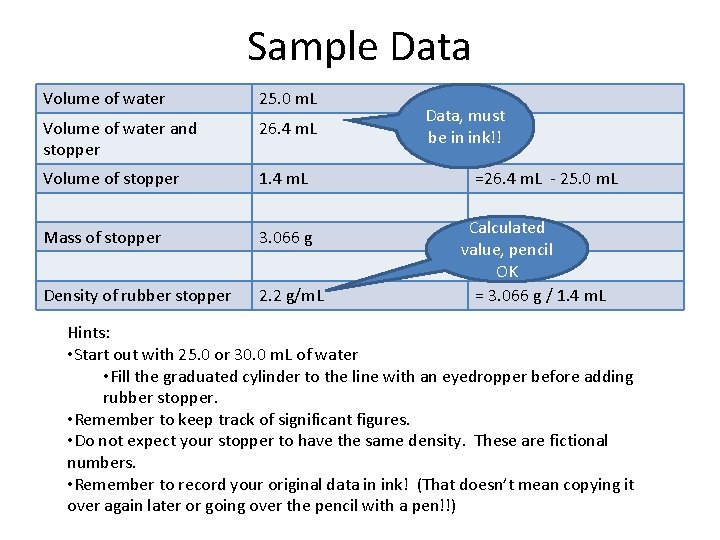
Sample Data Volume of water 25. 0 m. L Volume of water and stopper 26. 4 m. L Volume of stopper 1. 4 m. L Mass of stopper 3. 066 g Density of rubber stopper 2. 2 g/m. L Data, must be in ink!! =26. 4 m. L - 25. 0 m. L Calculated value, pencil OK = 3. 066 g / 1. 4 m. L Hints: • Start out with 25. 0 or 30. 0 m. L of water • Fill the graduated cylinder to the line with an eyedropper before adding rubber stopper. • Remember to keep track of significant figures. • Do not expect your stopper to have the same density. These are fictional numbers. • Remember to record your original data in ink! (That doesn’t mean copying it over again later or going over the pencil with a pen!!)
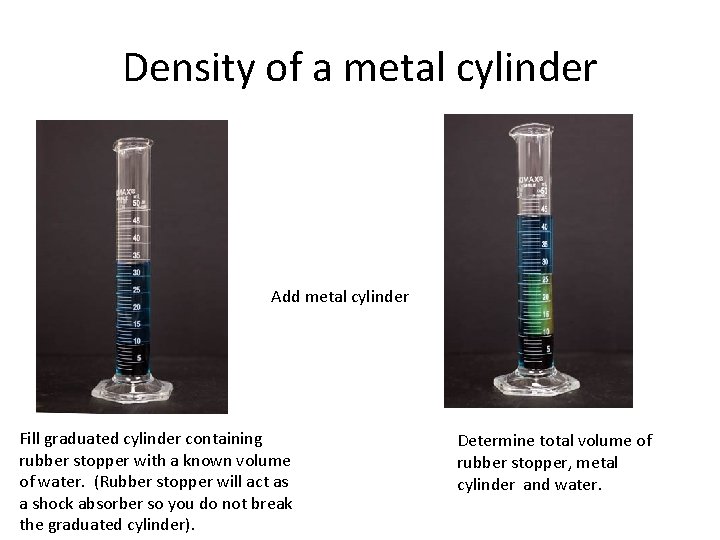
Density of a metal cylinder Add metal cylinder Fill graduated cylinder containing rubber stopper with a known volume of water. (Rubber stopper will act as a shock absorber so you do not break the graduated cylinder). Determine total volume of rubber stopper, metal cylinder and water.
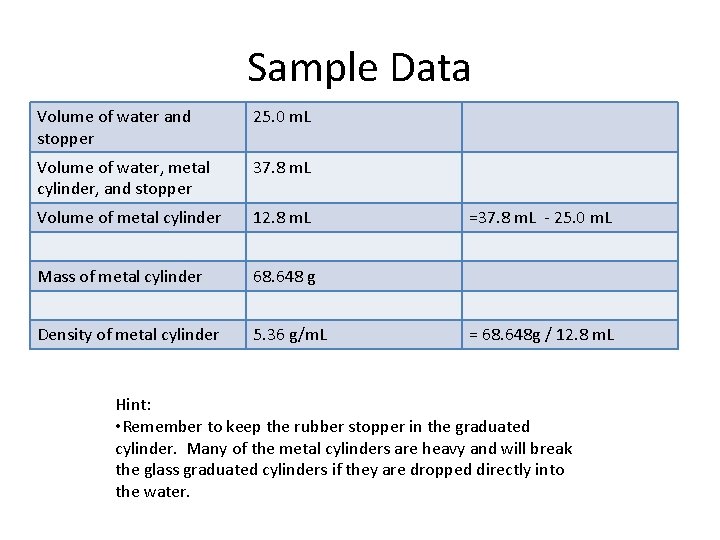
Sample Data Volume of water and stopper 25. 0 m. L Volume of water, metal cylinder, and stopper 37. 8 m. L Volume of metal cylinder 12. 8 m. L Mass of metal cylinder 68. 648 g Density of metal cylinder 5. 36 g/m. L =37. 8 m. L - 25. 0 m. L = 68. 648 g / 12. 8 m. L Hint: • Remember to keep the rubber stopper in the graduated cylinder. Many of the metal cylinders are heavy and will break the glass graduated cylinders if they are dropped directly into the water.

Density of an unknown liquid • Weigh and record the mass of a clean, dry beaker. • Rinse graduated cylinder with ~5 m. L of unknown liquid. Dispose of the rinse liquid. • (It may go down the sink. It is rubbing alcohol. ) • Fill the graduated cylinder with 20 -25 m. L of the unknown liquid. • Record the volume of the liquid. • Transfer the liquid to the weighed beaker. • Reweigh the beaker. • Repeat with a different volume of unknown liquid
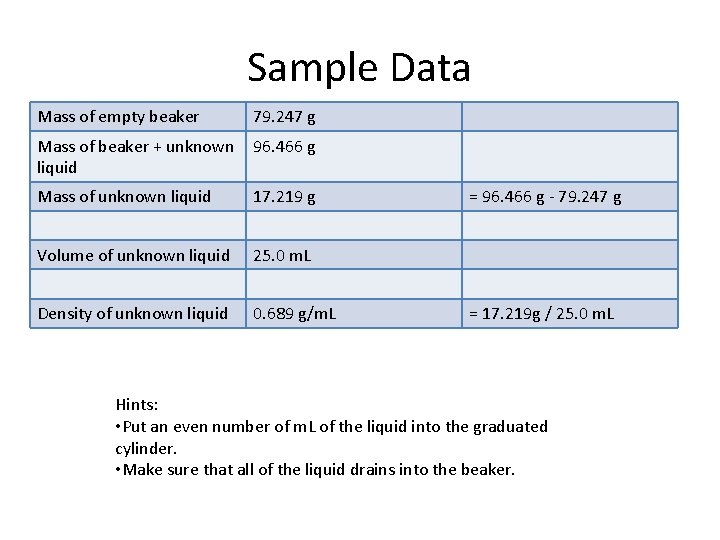
Sample Data Mass of empty beaker 79. 247 g Mass of beaker + unknown liquid 96. 466 g Mass of unknown liquid 17. 219 g Volume of unknown liquid 25. 0 m. L Density of unknown liquid 0. 689 g/m. L = 96. 466 g - 79. 247 g = 17. 219 g / 25. 0 m. L Hints: • Put an even number of m. L of the liquid into the graduated cylinder. • Make sure that all of the liquid drains into the beaker.

Finishing up • Once you have gathered all of the data, enter it onto the spreadsheet at http: //www. grossmont. edu/cwillard/lab. htm • Either print out the spreadsheet or get me to sign off on your lab sheet. • Answer all of the questions in the lab. Questions and sample calculations may be done in pencil. (Just be sure to record the data in ink!)

Due today • Experiment 2 from last week • Worksheet #1 from last week • If you leave early • Experiment #3 • Worksheet #2 • Have your lab data stamped before you leave!!
- Slides: 10