Density Density Two things contribute to density 1

Density

Density Two things contribute to density: 1. The 2. The mass of the atoms or molecules that make up the material. volume or amount of space the material takes up. If the molecules are “packed” more closely, the object will be more dense. Material that is denser has more matter, or STUFF packed into the same space. (Think NYC, densely populated. ) The more stuff that is in the nuclei of the atoms of the substance the greater the density will be.
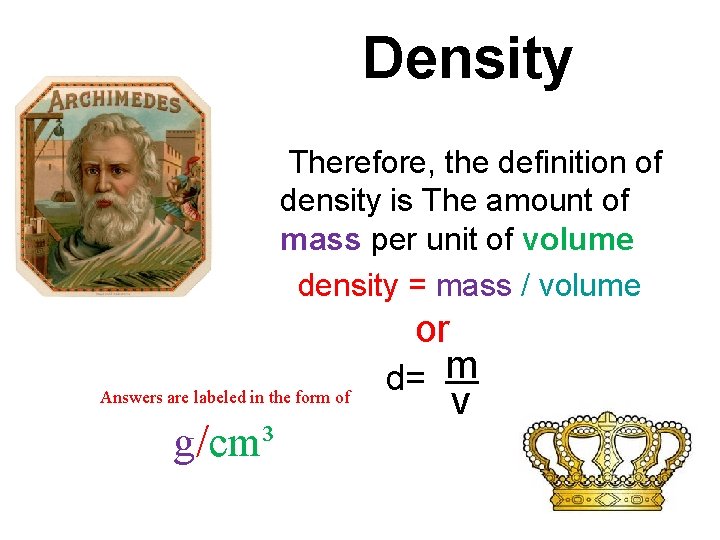
Density Therefore, the definition of density is The amount of mass per unit of volume density = mass / volume Answers are labeled in the form of g/cm³ or d= m v
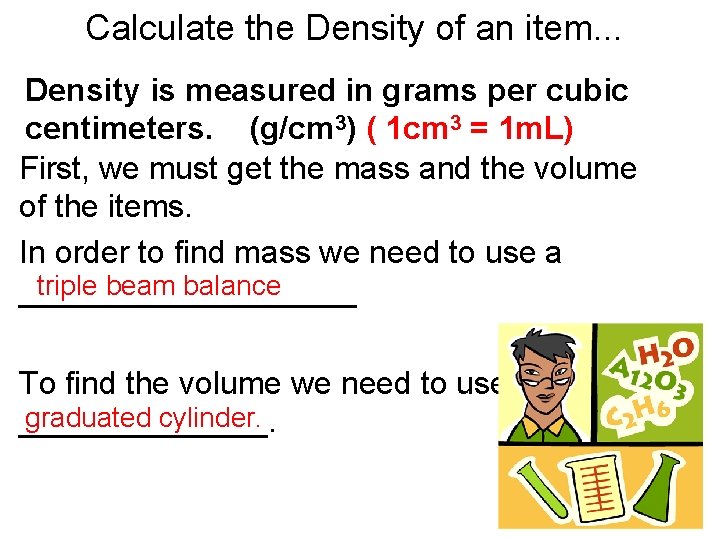
Calculate the Density of an item. . . Density is measured in grams per cubic centimeters. (g/cm 3) ( 1 cm 3 = 1 m. L) First, we must get the mass and the volume of the items. In order to find mass we need to use a triple beam balance __________ To find the volume we need to use a graduated cylinder. _______.

• Water has a density of 1. 00 g/cm³ • Any solid or liquid with a density greater than 1. 00 g/cm³ will sink in water • Any solid or liquid with a density less than 1. 00 g/cm³ will float in water Intro to Density (3: 12)
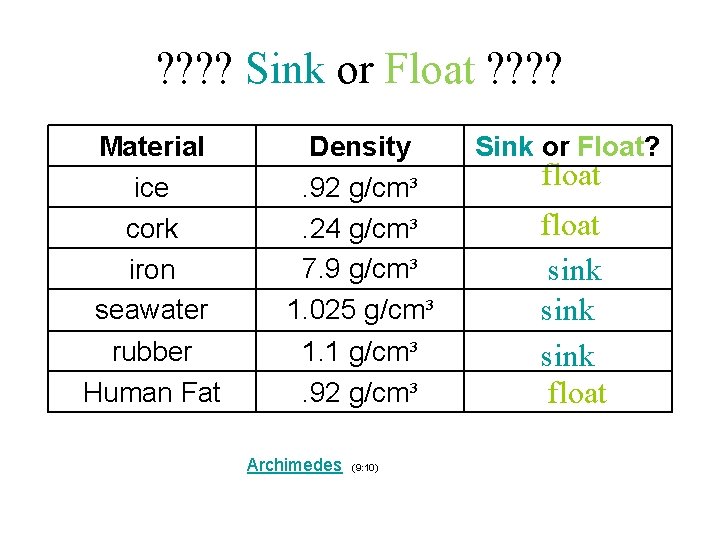
? ? Sink or Float ? ? Material ice cork iron seawater Density. 92 g/cm³. 24 g/cm³ 7. 9 g/cm³ 1. 025 g/cm³ rubber Human Fat 1. 1 g/cm³. 92 g/cm³ Archimedes (9: 10) Sink or Float? float sink float
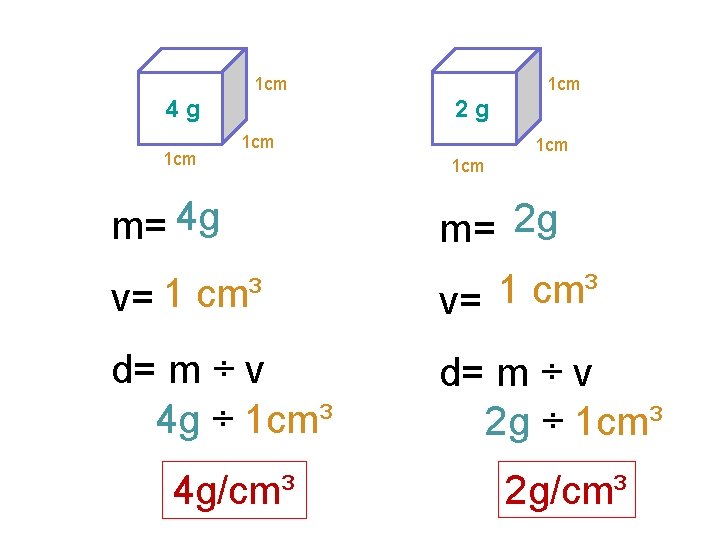
1 cm 2 g 4 g 1 cm 1 cm 1 cm m= 4 g m= 2 g v= 1 cm³ d= m ÷ v 4 g ÷ 1 cm³ d= m ÷ v 2 g ÷ 1 cm³ 4 g/cm³ 2 g/cm³
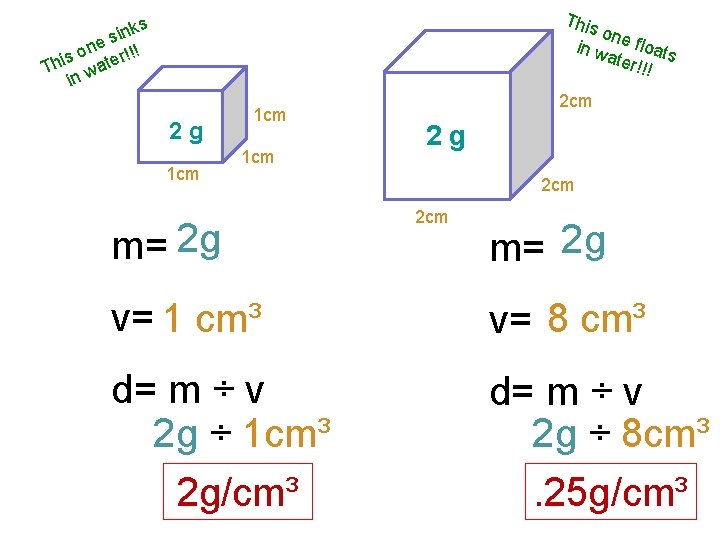
This on in w e floats ater !!! ks n i s ne r!!! o s Thi wate in 2 g 1 cm 1 cm m= 2 g 2 cm 2 cm m= 2 g v= 1 cm³ v= 8 cm³ d= m ÷ v 2 g ÷ 1 cm³ 2 g/cm³ d= m ÷ v 2 g ÷ 8 cm³. 25 g/cm³
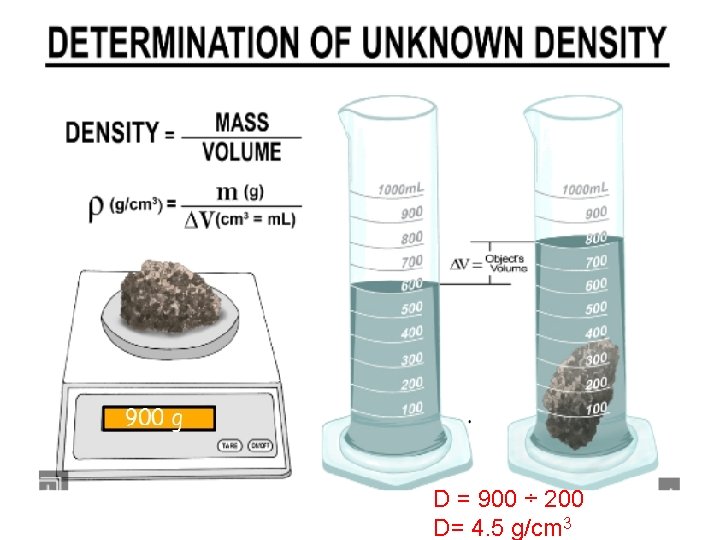
D = 900 ÷ 200 D= 4. 5 g/cm 3
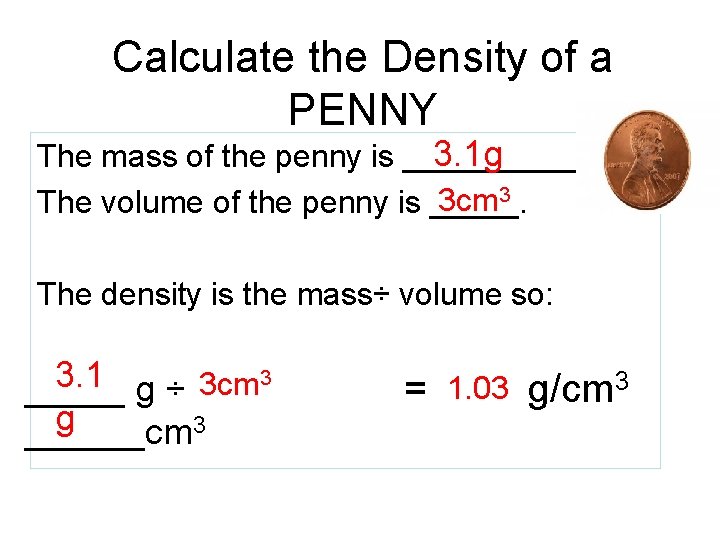
Calculate the Density of a PENNY 3. 1 g The mass of the penny is ______. 3 cm 3 The volume of the penny is _____. The density is the mass÷ volume so: 3 3. 1 3 cm _____ g ÷ g 3 ______cm = 1. 03 g/cm 3

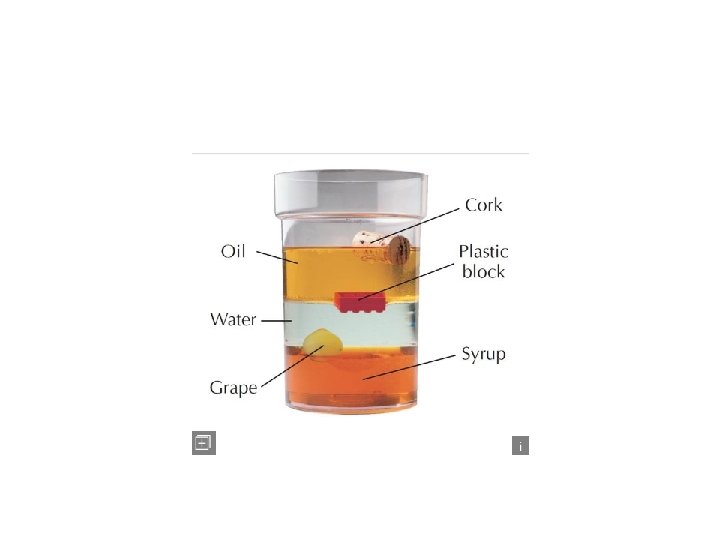
Why do you think all of these liquids are layered the way they are? Density The answer is Density (4: 43)
- Slides: 12