Density Definition the relationship between mass and volume
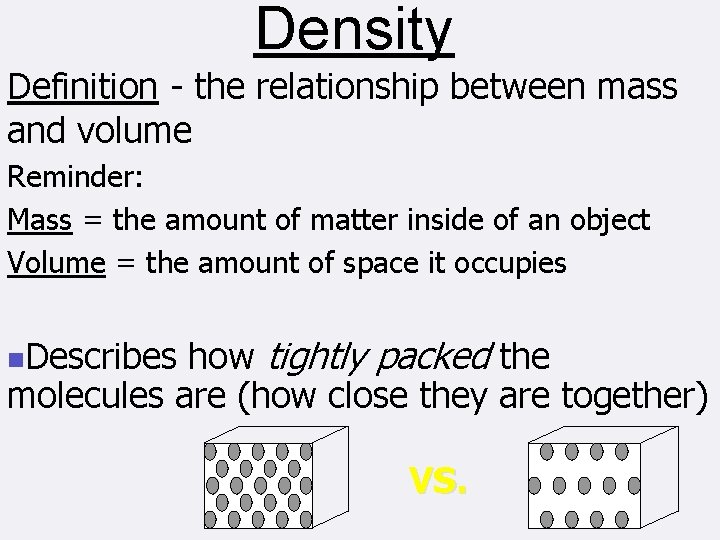
Density Definition - the relationship between mass and volume Reminder: Mass = the amount of matter inside of an object Volume = the amount of space it occupies how tightly packed the molecules are (how close they are together) n. Describes VS.

Not all solids will have the same density! n Because Lead has a lot of atoms squeezed together, it has a: n. High density n Because Wax has fewer molecules squeezed together, it has a: n. Low density
a solids that are Pure Substances or Homogeneous Mixtures, density will stay the same no matter how large or small the sample n For n. For example, the density of a tree is the same as the density of a cut piece of the same tree
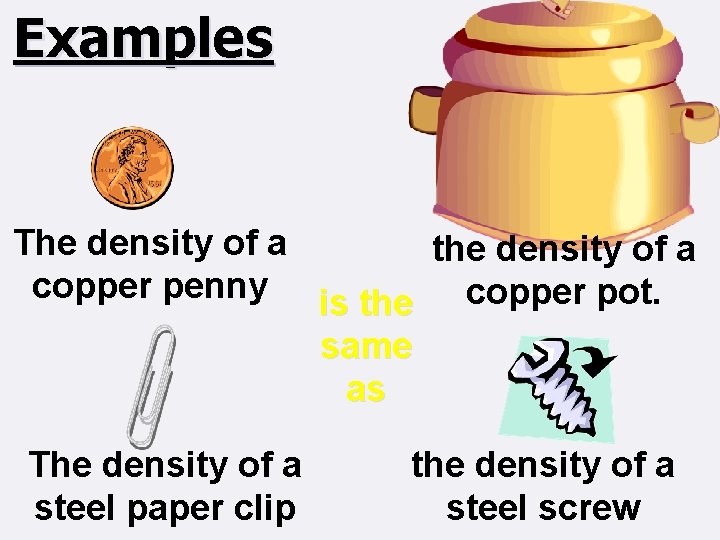
Examples The density of a the density of a copper penny is the copper pot. same as The density of a steel paper clip the density of a steel screw

n For solids that are Heterogeneous Mixtures, they will not always have the same density Not all pieces of the same chocolate chip cookie are going to have the same density n
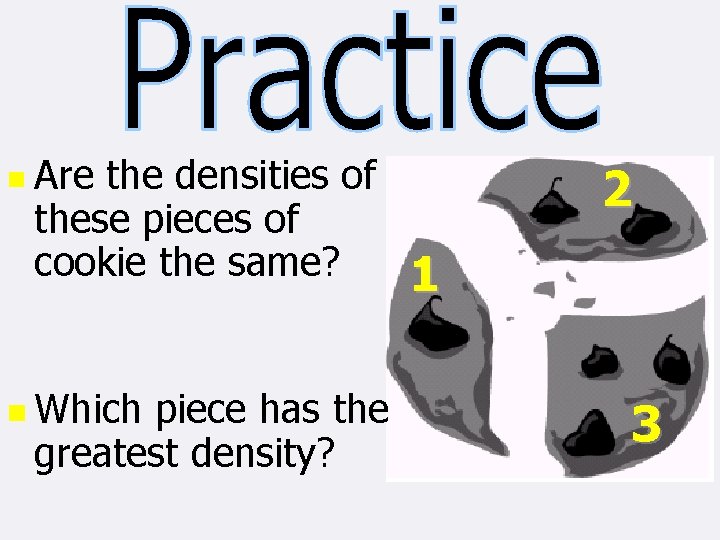
n Are the densities of these pieces of cookie the same? n Which piece has the greatest density? 2 1 3

n We can also examine how tightly packed the atoms are in Fluids. n Liquids and Gases have density based on their mass and volume.

A phase change from a Solid → Liquid or Liquid →Gas will cause density to change. (the opposite is true as well) q The Mass will not change with a phase change HOWEVER… q. Volume does change
This is why: o As heat energy is added, the particles inside a substance gain energy, spread out, and move around more. o Liquids and Gases tend to take up more space, because they can slide around!

In general, an increase in volume will result in a lower density Exception H 2 O is different!! In this case the solid (Ice) is less dense than the liquid (water) because of hydrogen bonding.
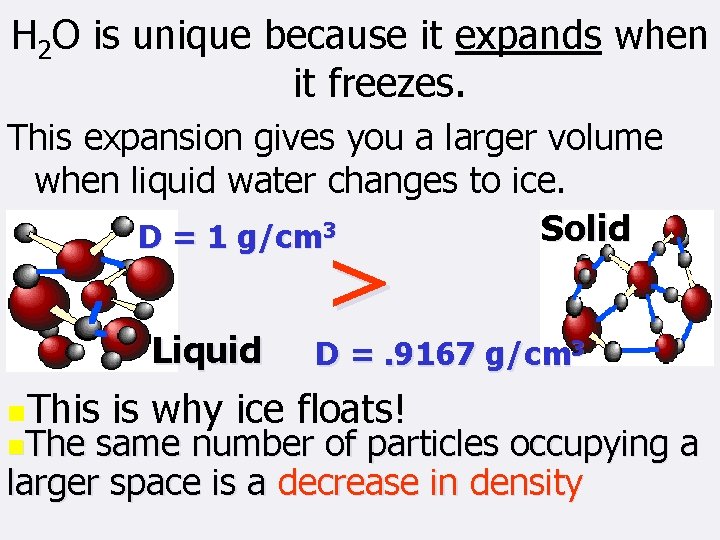
H 2 O is unique because it expands when it freezes. This expansion gives you a larger volume when liquid water changes to ice. Solid D = 1 g/cm 3 Liquid n. This n. The > D =. 9167 g/cm 3 is why ice floats! same number of particles occupying a larger space is a decrease in density

What would happen if ice was more dense than water? n. Ice would sink to bottoms of lakes, rivers and oceans n. It wouldn’t melt in the summer at the bottom.
- Slides: 12