Density d Calculations PM m is the mass

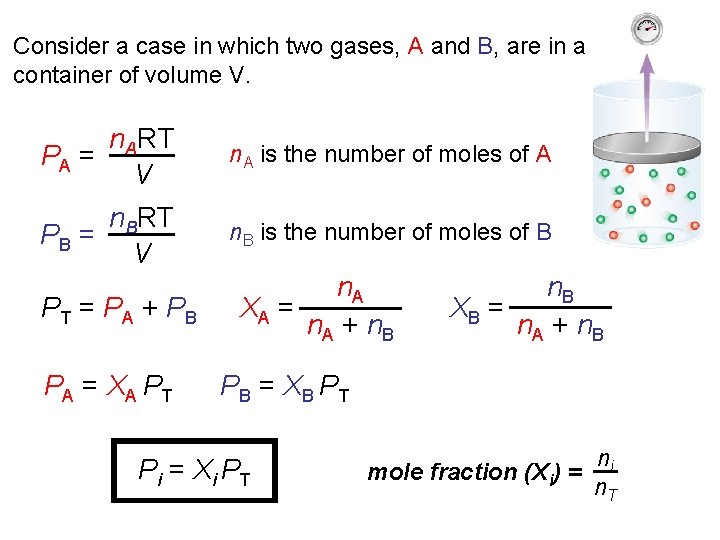

- Slides: 14


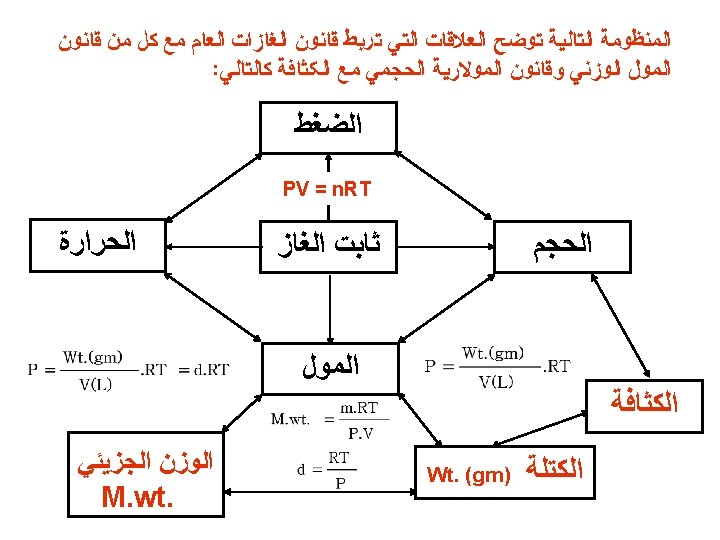
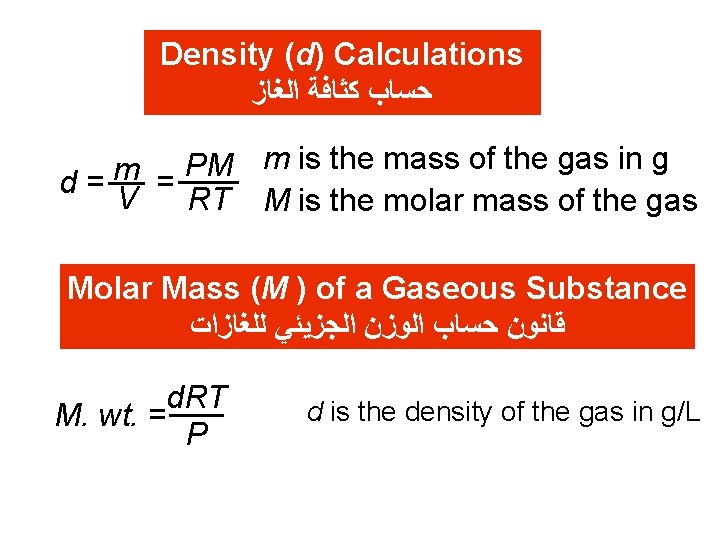
Density (d) Calculations ﺍﻟﻐﺎﺯ ﻛﺜﺎﻓﺔ ﺣﺴﺎﺏ PM m is the mass of the gas in g m d= V = RT M is the molar mass of the gas Molar Mass (M ) of a Gaseous Substance ﻟﻠﻐﺎﺯﺍﺕ ﺍﻟﺠﺰﻳﺌﻲ ﺍﻟﻮﺯﻥ ﺣﺴﺎﺏ ﻗﺎﻧﻮﻥ d. RT M. wt. = P d is the density of the gas in g/L

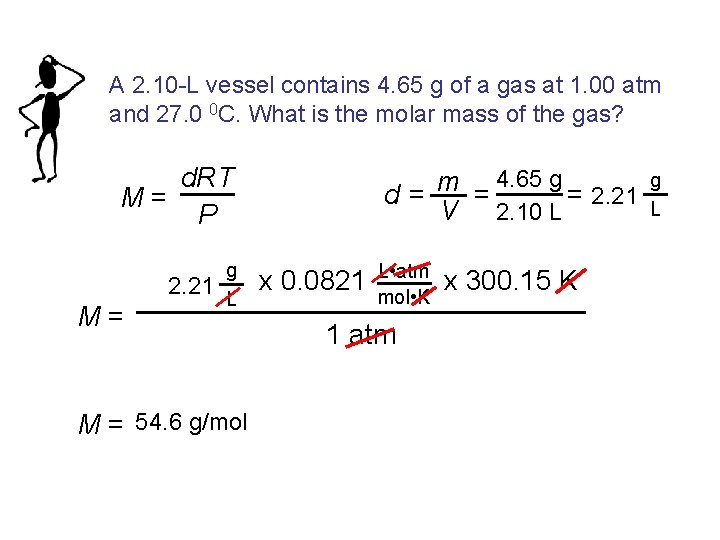
A 2. 10 -L vessel contains 4. 65 g of a gas at 1. 00 atm and 27. 0 0 C. What is the molar mass of the gas? d. RT M= P 2. 21 M= g L M = 54. 6 g/mol 4. 65 g m = = 2. 21 d= V 2. 10 L x 0. 0821 L • atm mol • K 1 atm x 300. 15 K g L

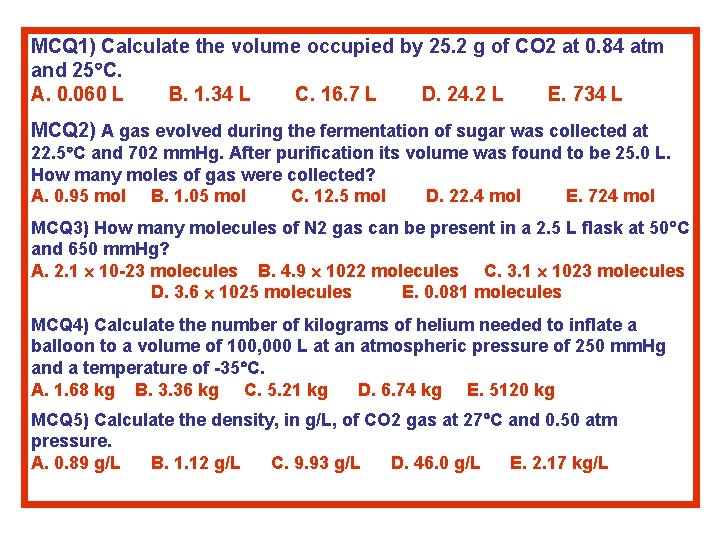
MCQ 1) Calculate the volume occupied by 25. 2 g of CO 2 at 0. 84 atm and 25 C. A. 0. 060 L B. 1. 34 L C. 16. 7 L D. 24. 2 L E. 734 L MCQ 2) A gas evolved during the fermentation of sugar was collected at 22. 5 C and 702 mm. Hg. After purification its volume was found to be 25. 0 L. How many moles of gas were collected? A. 0. 95 mol B. 1. 05 mol C. 12. 5 mol D. 22. 4 mol E. 724 mol MCQ 3) How many molecules of N 2 gas can be present in a 2. 5 L flask at 50 C and 650 mm. Hg? A. 2. 1 10 -23 molecules B. 4. 9 1022 molecules C. 3. 1 1023 molecules D. 3. 6 1025 molecules E. 0. 081 molecules MCQ 4) Calculate the number of kilograms of helium needed to inflate a balloon to a volume of 100, 000 L at an atmospheric pressure of 250 mm. Hg and a temperature of -35 C. A. 1. 68 kg B. 3. 36 kg C. 5. 21 kg D. 6. 74 kg E. 5120 kg MCQ 5) Calculate the density, in g/L, of CO 2 gas at 27 C and 0. 50 atm pressure. A. 0. 89 g/L B. 1. 12 g/L C. 9. 93 g/L D. 46. 0 g/L E. 2. 17 kg/L

MCQ 6) Which of the following gases will have the greatest density at the same specified temperature and pressure? A. H 2 B. CCl. F 3 C. CO 2 D. C 2 H 6 E. CF 4 MCQ 7) Which one of the following gases is "lighter-than-air"? A. Cl 2 B. SO 2 C. PH 3 D. NO 2 E. Ne MCQ 8) Two moles of chlorine gas at 20. 0 C are heated to 350 C while the volume is kept constant. The density of the gas A. increases. B. decreases. C. remains the same. D. Not enough information is given to correctly answer the question. MCQ 9) What is the molar mass of Freon-11 gas if its density is 6. 13 g/L at STP? A. 0. 274 g/mol B. 3. 644 g/mol C. 78. 20 g/mol D. 137. 4 g/mol E. 365. 0 g/mol MCQ 10) A 0. 271 g sample of an unknown vapor occupies 294 m. L at 140 C and 847 mm. Hg. The empirical formula of the compound is CH 2. What is the molecular formula of the compound? A. CH 2 B. C 2 H 4 C. C 3 H 6 D. C 4 H 8 E. C 6 H 12

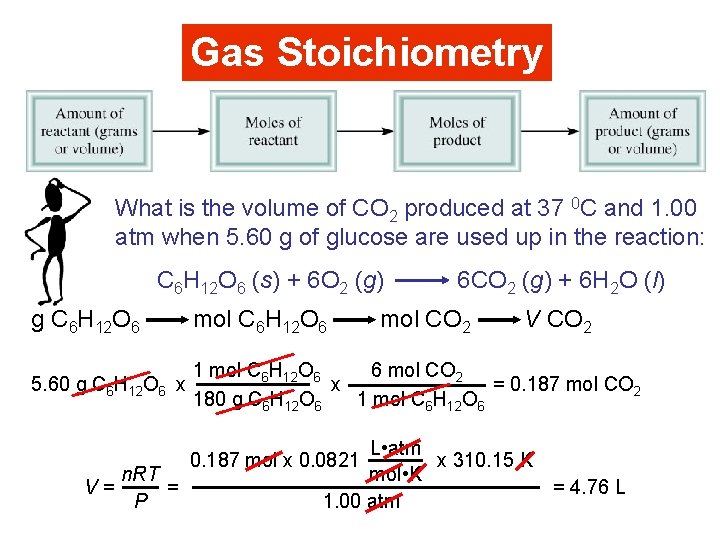
Gas Stoichiometry What is the volume of CO 2 produced at 37 0 C and 1. 00 atm when 5. 60 g of glucose are used up in the reaction: C 6 H 12 O 6 (s) + 6 O 2 (g) 6 CO 2 (g) + 6 H 2 O (l) g C 6 H 12 O 6 mol C 6 H 12 O 6 5. 60 g C 6 H 12 O 6 x 6 mol CO 2 1 mol C 6 H 12 O 6 x = 0. 187 mol CO 2 180 g C 6 H 12 O 6 1 mol C 6 H 12 O 6 V= n. RT = P mol CO 2 V CO 2 L • atm x 310. 15 K mol • K 1. 00 atm 0. 187 mol x 0. 0821 = 4. 76 L

ﺍﻟﻐﺎﺯﻳﺔ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺍﻟﺤﺴﺎﺑﺎﺕ ﺗﺸﻤﻞ ﺗﻤﺎﺭﻳﻦ ( ﺍﻟﺨﺎﻣﺲ )ﺍﻟﺒﺎﺏ ﺍﻟﻐﺎﺯﺍﺕ ﻣﻊ ( ﻭﺍﻟﺮﺍﺑﻊ ﺍﻟﺜﺎﻟﺚ )ﺍﻟﺒﺎﺏ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺣﺴﺎﺑﺎﺕ ﺑﻴﻦ ﻣﺎ ﺗﺠﻤﻊ MCQ 11) A gaseous compound is 30. 4% nitrogen and 69. 6% oxygen by mass. A 5. 25 -g sample of the gas occupies a volume of 1. 00 L and exerts a pressure of 1. 26 atm at -4. 0 C. Which of the following is its molecular formula? A. NO B. NO 2 C. N 3 O 6 D. N 2 O 4 E. N 2 O 5 MCQ 12) A sample of hydrogen gas was collected over water at 21 C and 685 mm. Hg. The volume of the container was 7. 80 L. Calculate the mass of H 2(g) collected. (Vapor pressure of water = 18. 6 mm. Hg at 21 C. ) A. 0. 283 g B. 0. 567 g C. 0. 589 g D. 7. 14 g E. 435 g MCQ 13) A sample of carbon monoxide gas was collected in a 2. 0 L flask by displacing water at 28 C and 810 mm. Hg. Calculate the number of CO molecules in the flask. The vapor pressure of water at 28 C is 28. 3 mm. Hg. A. 5. 0 1022 B. 5. 2 1022 C. 3. 8 1023 D. 5. 4 1023 E. 3. 8 1025 MCQ 14) What volume of oxygen gas at 320 K and 680 torr will react completely with 2. 50 L of NO gas at the same temperature and pressure? 2 NO(g) + O 2(g) 2 NO 2(g) A. 1. 25 L B. 2. 50 L C. 3. 00 L D. 1. 00 L E. 5. 00 L

MCQ 15) What volume of CO 2 gas at 645 torr and 800 K could be produced by the reaction of 45 g of Ca. CO 3 according to the equation? Ca. CO 3(s) Ca. O(s) + CO 2(g) A. 0. 449 L B. 22. 4 L C. 25. 0 L D. 34. 8 L E. 45. 7 m. L MCQ 16) Chlorine gas can be prepared in the laboratory by the reaction of solid manganese dioxide with hydrochloric acid. (The other reaction products are aqueous manganese chloride and water. ) How much Mn. O 2 should be added to excess HCl to obtain 275 m. L of chlorine gas at 5. 0 C and 650 mm. Hg? A. 1. 18 10 -4 g B. 0. 896 g C. 1. 22 g D. 49. 8 g E. 8, 440 g MCQ 17) What mass of KCl. O 3 must be decomposed to produce 126 L of oxygen gas at 133 C and 0. 880 atm? (The other reaction product is solid KCl. ) A. 24. 6 g B. 70. 8 g C. 272 g D. 408 g E. 612 g MCQ 18)


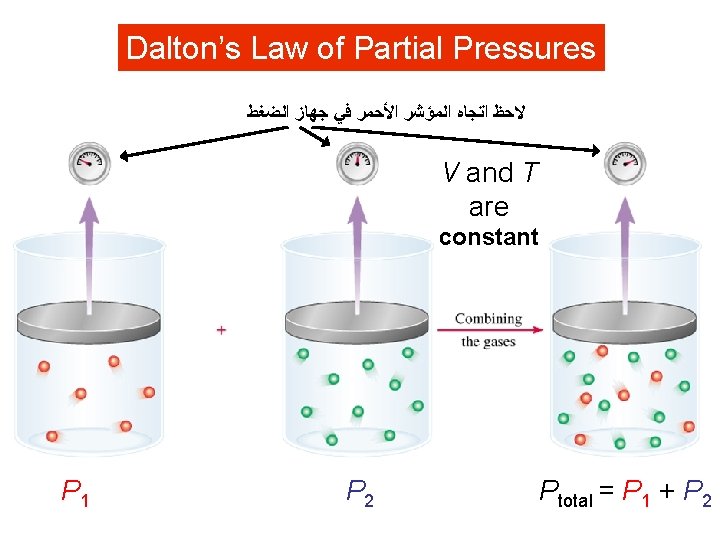
Dalton’s Law of Partial Pressures ﺍﻟﻀﻐﻂ ﺟﻬﺎﺯ ﻓﻲ ﺍﻷﺤﻤﺮ ﺍﻟﻤﺆﺸﺮ ﺍﺗﺠﺎﻩ ﻻﺣﻆ V and T are constant P 1 P 2 Ptotal = P 1 + P 2
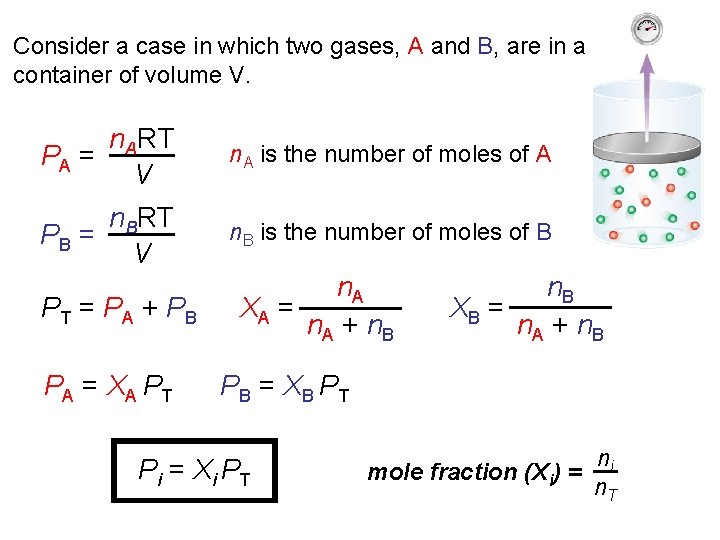
Consider a case in which two gases, A and B, are in a container of volume V. n. ART PA = V n. A is the number of moles of A n. BRT PB = V n. B is the number of moles of B PT = PA + PB PA = XA PT n. A XA = n. A + n. B XB = n. A + n. B PB = XB PT Pi = Xi PT mole fraction (Xi) = ni n. T

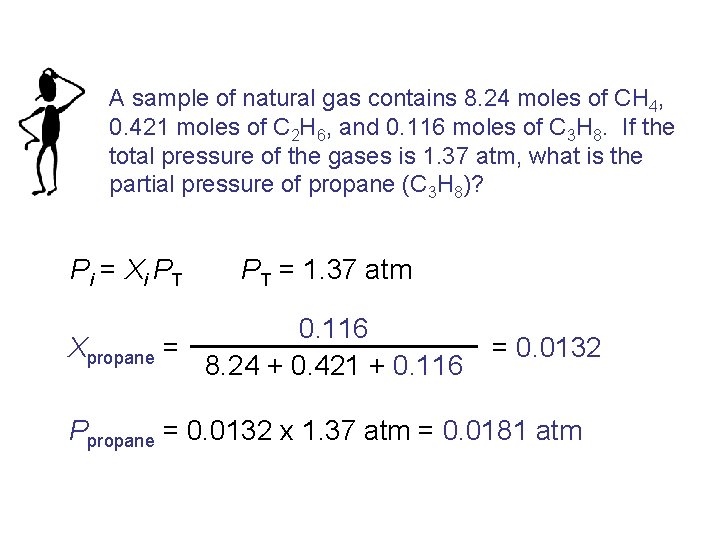
A sample of natural gas contains 8. 24 moles of CH 4, 0. 421 moles of C 2 H 6, and 0. 116 moles of C 3 H 8. If the total pressure of the gases is 1. 37 atm, what is the partial pressure of propane (C 3 H 8)? Pi = Xi PT PT = 1. 37 atm 0. 116 Xpropane = 8. 24 + 0. 421 + 0. 116 = 0. 0132 Ppropane = 0. 0132 x 1. 37 atm = 0. 0181 atm


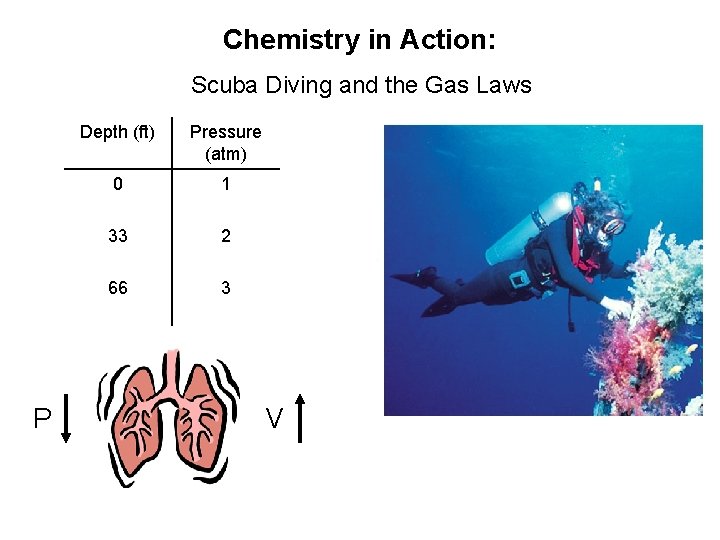
Chemistry in Action: Scuba Diving and the Gas Laws P Depth (ft) Pressure (atm) 0 1 33 2 66 3 V