DENSITY BUTTONS Click us Clicking here will allow
DENSITY BUTTONS Click us: Clicking here will allow you to. SI hear some information on on the Clicking on me take you to multipliers table. Clicking on me will take you to a list of equations, clicking Clicking here will reveal some information. Clicking here will reveal an answer. Clicking here will move you back a page. Clicking here will bring back to this page. Clicking here will move youstop toyou the page. topic. Clicking here again thenext sound. on me again return youwill to previous page. me again will take you back toyour the previous page. TOPICS … density and molecules (2 pages). … the equation. … how to measure density. … practice questions (4 pages) …exam questions (3 pages).
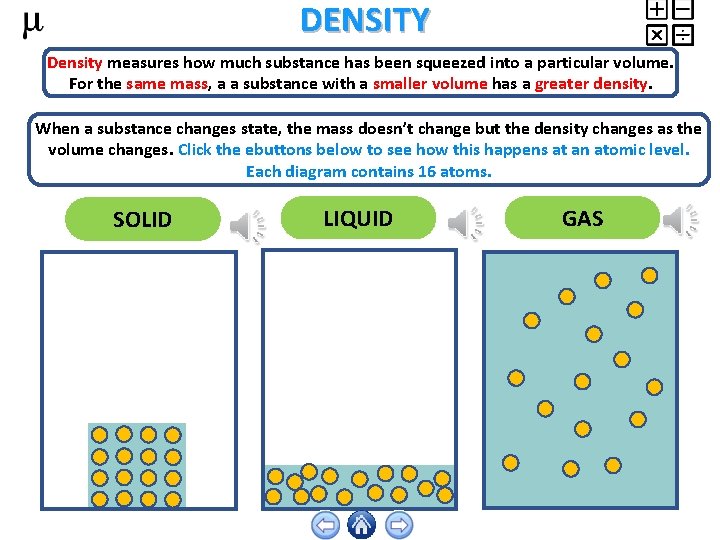
DENSITY Density measures how much substance has been squeezed into a particular volume. For the same mass, a a substance with a smaller volume has a greater density. When a substance changes state, the mass doesn’t change but the density changes as the volume changes. Click the ebuttons below to see how this happens at an atomic level. Each diagram contains 16 atoms. SOLID LIQUID GAS
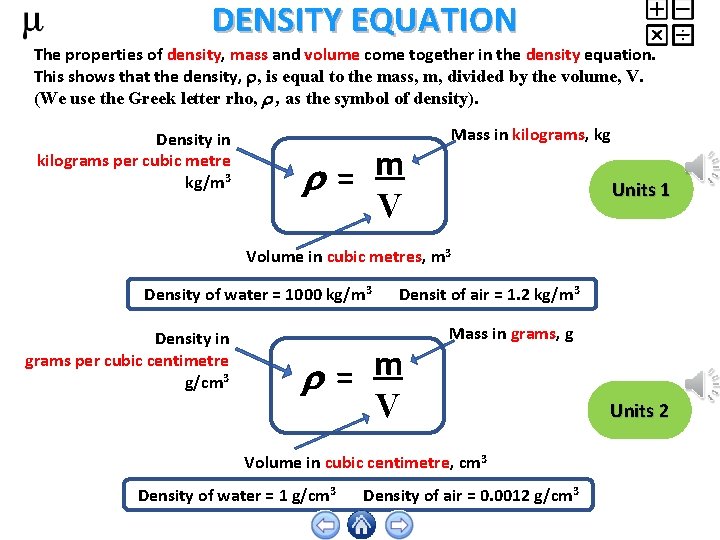
DENSITY EQUATION The properties of density, mass and volume come together in the density equation. This shows that the density, , is equal to the mass, m, divided by the volume, V. (We use the Greek letter rho, , as the symbol of density). Density in kilograms per cubic metre kg/m 3 m = V Mass in kilograms, kg Units 1 Volume in cubic metres, m 3 Density of water = 1000 kg/m 3 Density in grams per cubic centimetre g/cm 3 Densit of air = 1. 2 kg/m 3 m = V Mass in grams, g Volume in cubic centimetre, cm 3 Density of water = 1 g/cm 3 Density of air = 0. 0012 g/cm 3 Units 2
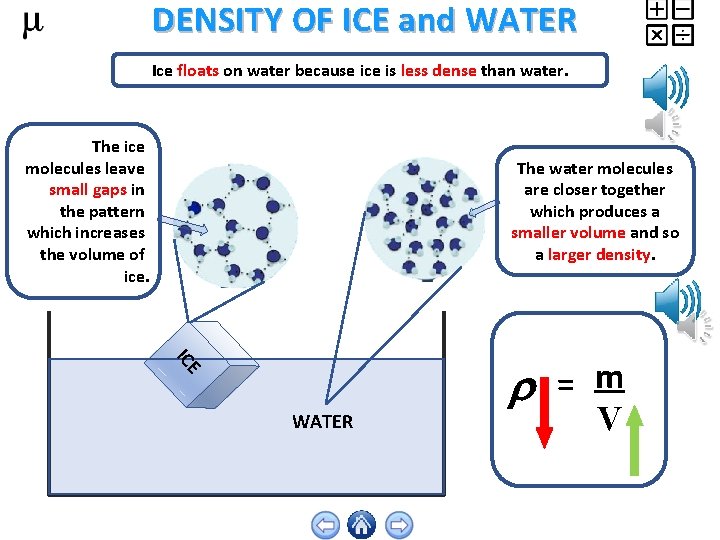
DENSITY OF ICE and WATER Ice floats on water because ice is less dense than water. The ice molecules leave small gaps in the pattern which increases the volume of ice. The water molecules are closer together which produces a smaller volume and so a larger density. E IC WATER = m V
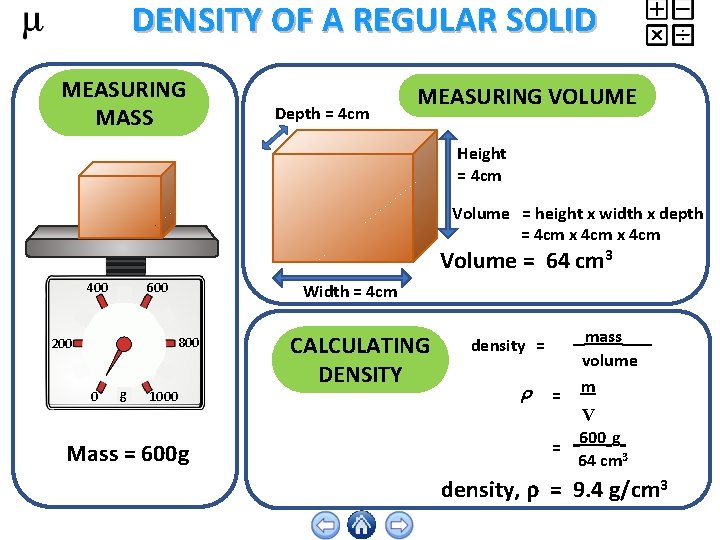
DENSITY OF A REGULAR SOLID MEASURING MASS Depth = 4 cm MEASURING VOLUME Height = 4 cm Volume = height x width x depth = 4 cm x 4 cm Volume = 64 cm 3 400 600 Width = 4 cm 800 200 0 g 1000 Mass = 600 g CALCULATING DENSITY density = = mass volume m V 600 g = 64 cm 3 density, = 9. 4 g/cm 3
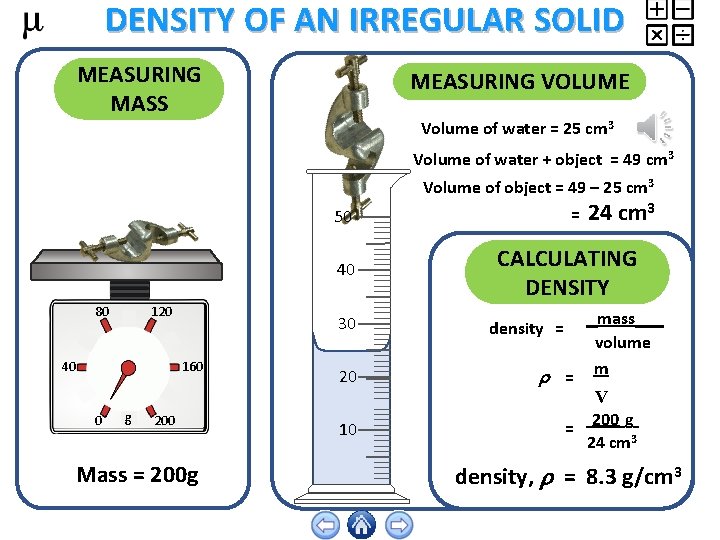
DENSITY OF AN IRREGULAR SOLID MEASURING MASS MEASURING VOLUME Volume of water = 25 cm 3 Volume of water + object = 49 cm 3 Volume of object = 49 – 25 cm 3 = 50 40 120 80 40 30 160 0 g 200 Mass = 200 g 20 10 24 cm 3 CALCULATING DENSITY density = = mass volume m V 200 g = 24 cm 3 density, = 8. 3 g/cm 3
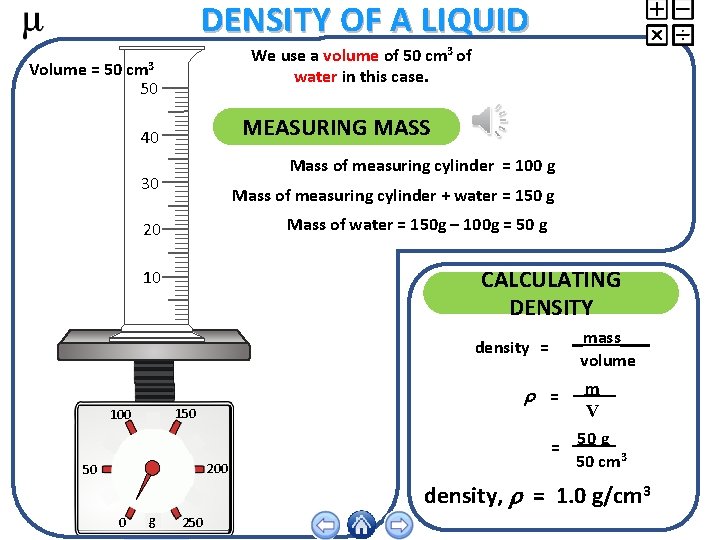
DENSITY OF A LIQUID We use a volume of 50 cm 3 of water in this case. Volume = 50 cm 3 50 MEASURING MASS 40 Mass of measuring cylinder = 100 g 30 Mass of measuring cylinder + water = 150 g Mass of water = 150 g – 100 g = 50 g 20 CALCULATING DENSITY 10 mass volume density = 150 100 200 50 m V 50 g = 50 cm 3 = density, = 1. 0 g/cm 3 0 g 250
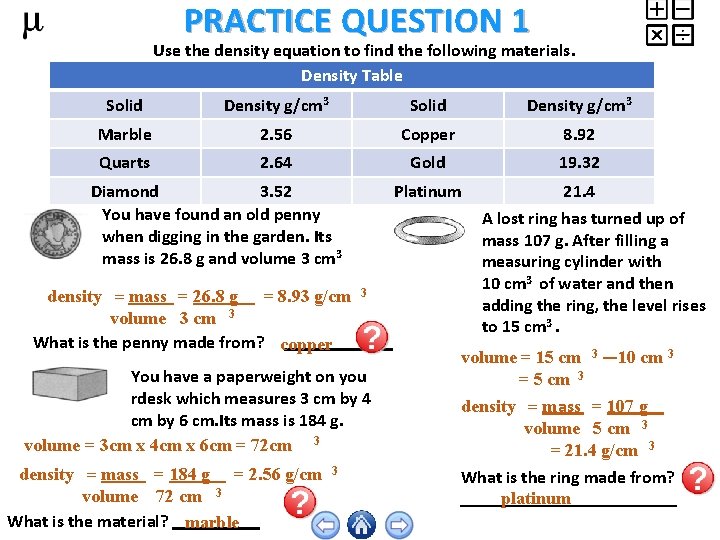
PRACTICE QUESTION 1 Use the density equation to find the following materials. Density Table Solid Density g/cm 3 Marble 2. 56 Copper 8. 92 Quarts 2. 64 Gold 19. 32 Platinum 21. 4 Diamond 3. 52 You have found an old penny when digging in the garden. Its mass is 26. 8 g and volume 3 cm 3 density = mass = 26. 8 g = 8. 93 g/cm volume 3 cm 3 What is the penny made from? copper 3 You have a paperweight on you rdesk which measures 3 cm by 4 cm by 6 cm. Its mass is 184 g. volume = 3 cm x 4 cm x 6 cm = 72 cm 3 density = mass = 184 g = 2. 56 g/cm volume 72 cm 3 What is the material? marble 3 A lost ring has turned up of mass 107 g. After filling a measuring cylinder with 10 cm 3 of water and then adding the ring, the level rises to 15 cm 3. volume = 15 cm 3 — 10 cm 3 = 5 cm 3 density = mass = 107 g volume 5 cm 3 = 21. 4 g/cm 3 What is the ring made from? platinum
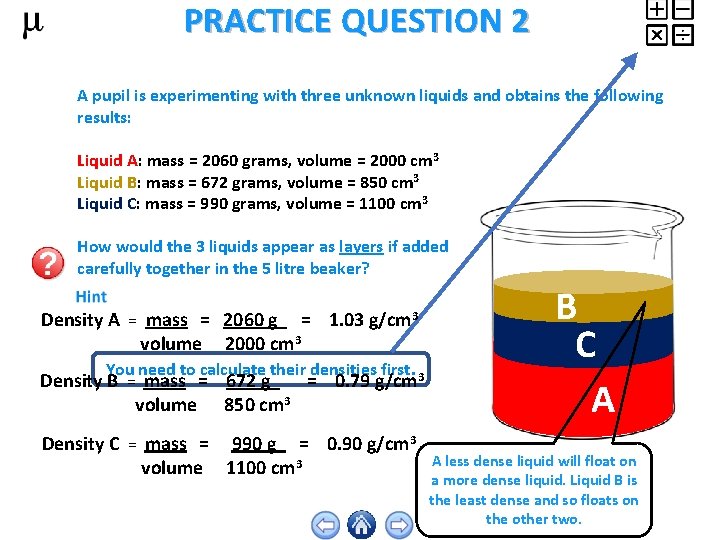
PRACTICE QUESTION 2 A pupil is experimenting with three unknown liquids and obtains the following results: Liquid A: mass = 2060 grams, volume = 2000 cm 3 Liquid B: mass = 672 grams, volume = 850 cm 3 Liquid C: mass = 990 grams, volume = 1100 cm 3 How would the 3 liquids appear as layers if added carefully together in the 5 litre beaker? Density A = mass = 2060 g = 1. 03 g/cm 3 volume 2000 cm 3 You need to calculate their densities first. Density B = mass = 672 g = 0. 79 g/cm 3 volume 850 cm 3 Density C = mass = 990 g = 0. 90 g/cm 3 volume 1100 cm 3 B C A A less dense liquid will float on a more dense liquid. Liquid B is the least dense and so floats on the other two.
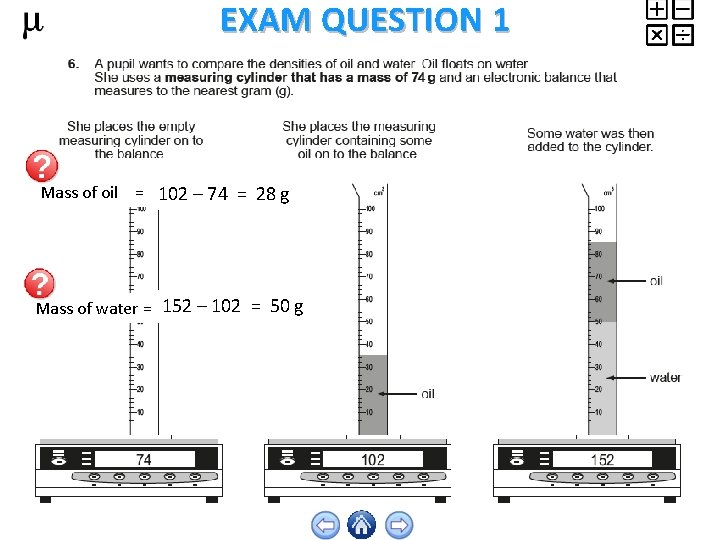
EXAM QUESTION 1 Mass of oil = 102 – 74 = 28 g Mass of water = 152 – 102 = 50 g
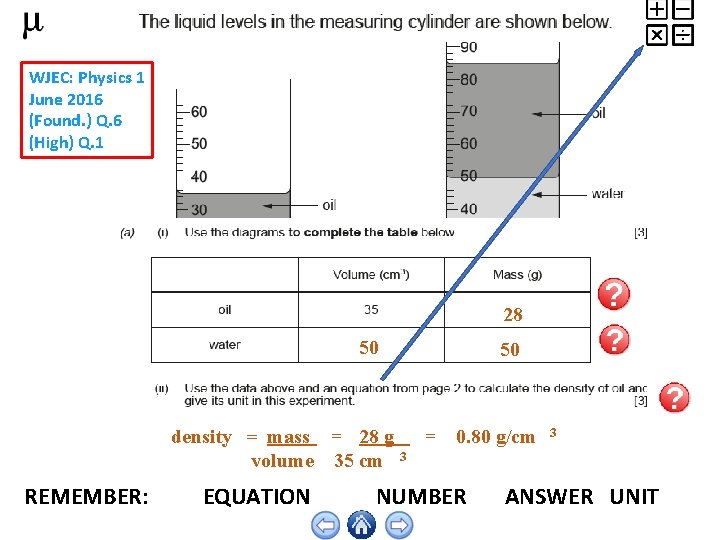
WJEC: Physics 1 June 2016 (Found. ) Q. 6 (High) Q. 1 28 50 density = mass = 28 g volume 35 cm REMEMBER: EQUATION 50 = 0. 80 g/cm 3 3 NUMBER ANSWER UNIT

EXAM QUESTION 2 - Higher WJEC: Physics 1 Jan 2016 (Higher) Q. 6 24 turbines Volume of water from one turbine = 700 m 3 per second (in table) Max volume of water from the barrage = 700 x 24 = 16, 800 m =m V m = x V = 1000 x 16, 800 = 1. 68 x 10 REMEMBER: EQUATION REARRANGE NUMBERS 3 /s 7 kg/s ANSWER UNIT

EXAM QUESTION 2 Maximum power = 24 x 10 MW = 240 MW P = V x I You can use 240, 000 watt and 3, 500 volts here. I = P = 240 MW = 240 x 10 6 = 6. 86 x 10 V 3. 5 k. V 3. 5 x 10 3 4 = 68. 6 k. A 68, 600 % efficiency = useful power output x 100% total power input = 10 MW x 100% = 62. 5% 16 MW As both values are in megawatts there is no need to change them. 62. 5%
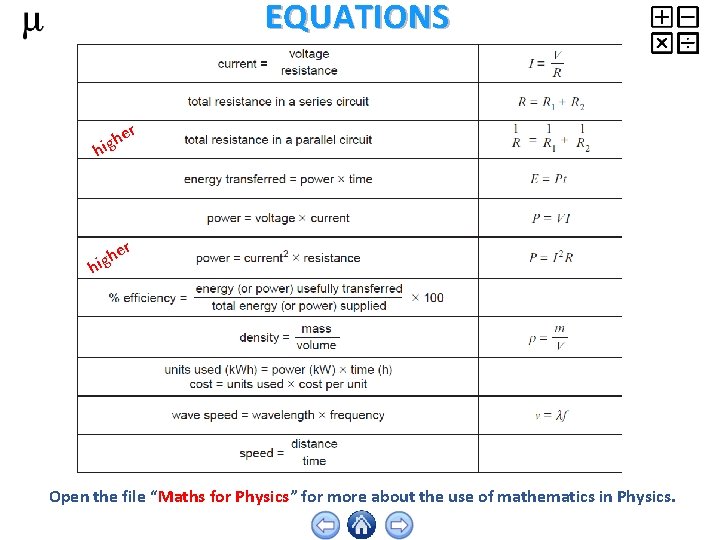
EQUATIONS r e igh h er h hig Open the file “Maths for Physics” for more about the use of mathematics in Physics.
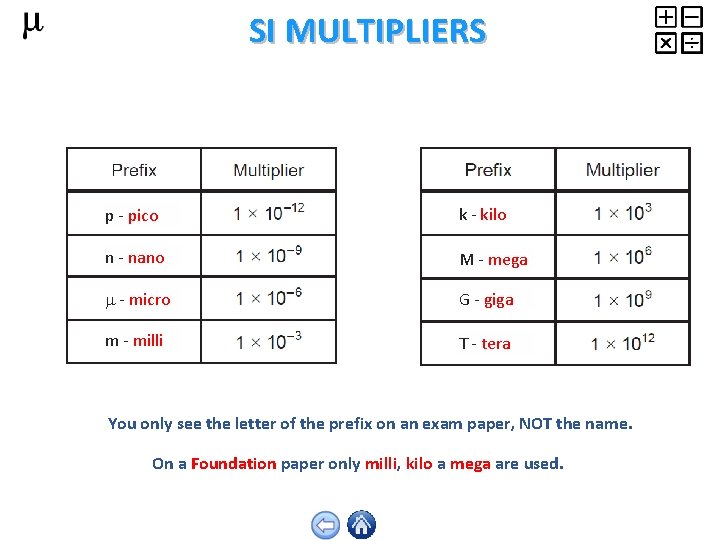
SI MULTIPLIERS p - pico k - kilo n - nano M - mega - micro G - giga m - milli T - tera You only see the letter of the prefix on an exam paper, NOT the name. On a Foundation paper only milli, kilo a mega are used.
- Slides: 15