DENSITY AND TEMPERATURE What is Density What is






- Slides: 11

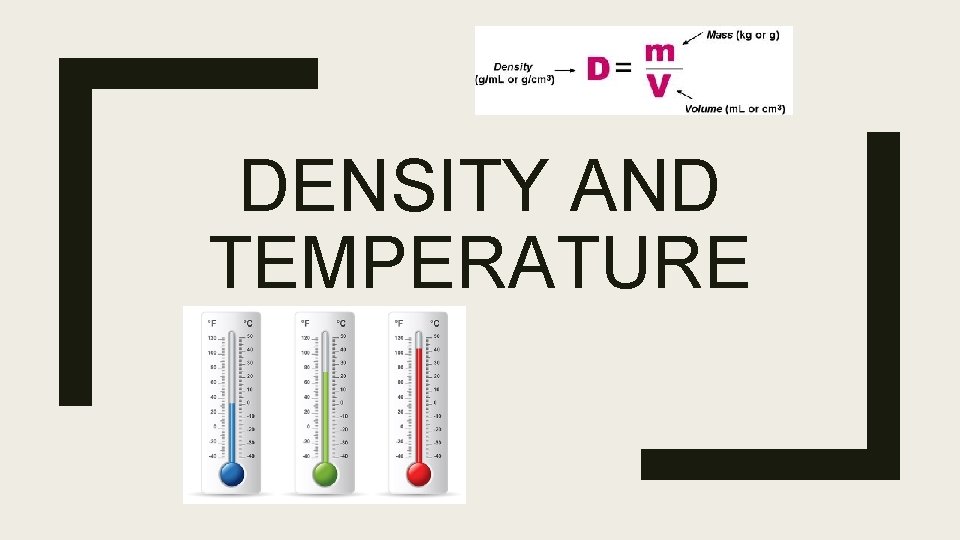
DENSITY AND TEMPERATURE

What is Density? ?

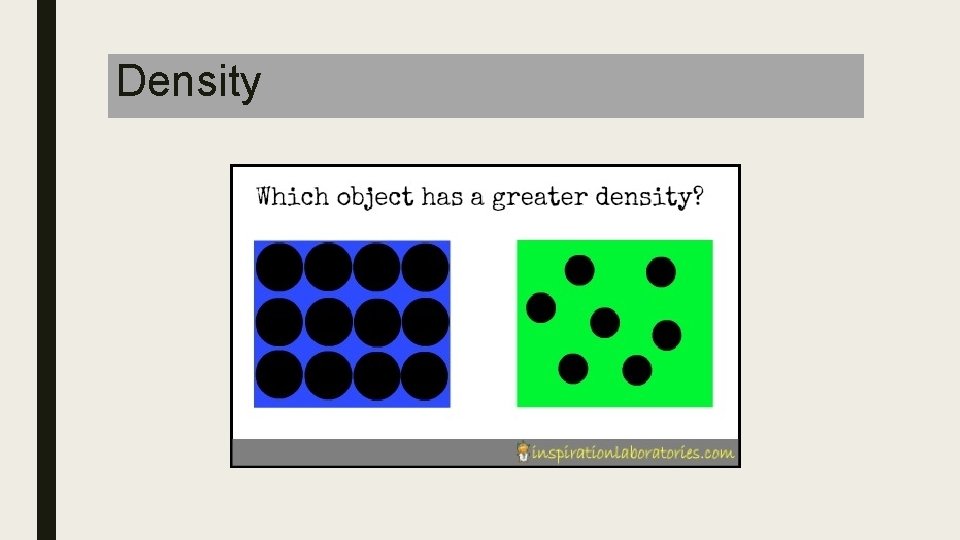
What is density? ■ Density is mass per unit volume of a substance ■ Ice floats in water because Ice is less dense than water ■ Liquids with low densities will float on top of substances that are more dense ■ Density= Mass/Volume – Units: ■ Mass grams (g) ■ Volume milliliters (m. L) or Cubic centimeters (cm 3)

Density

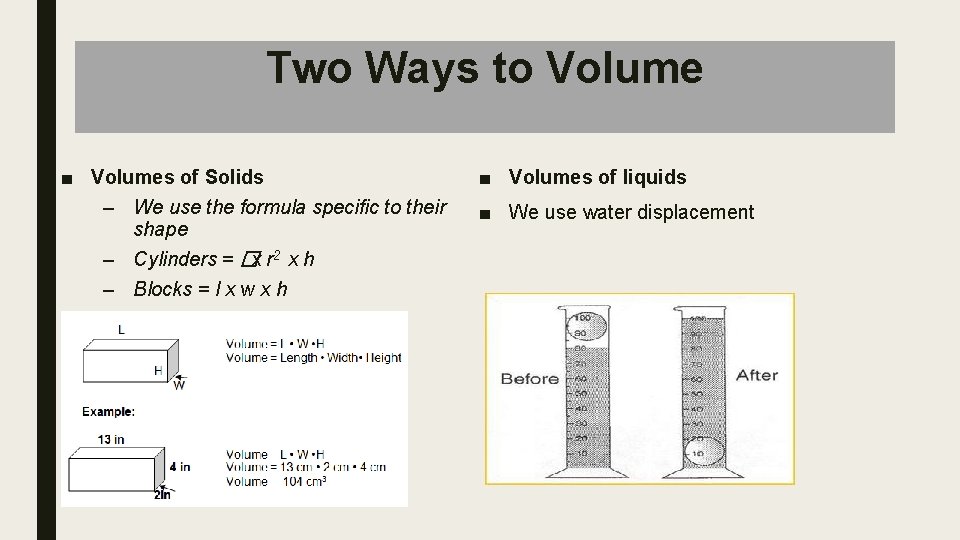
Two Ways to Volume ■ Volumes of Solids – We use the formula specific to their shape – Cylinders = �x r 2 x h – Blocks = l x w x h ■ Volumes of liquids ■ We use water displacement

Sample Problem 1 ■ Calculate the density of a material that has a mass of 52. 457 g and a volume of 13. 5 cm 3. ■ D = ? ? ? ■ M = 52. 457 g ■ V = 13. 5 cm 3 ■ D = 52. 457/13. 5 ■ D= 3. 8857 3. 89 g/cm 3

Sample Problem 2 ■ The density of silver is 10. 49 g/cm 3. If a sample of pure silver has a volume of 12. 993 cm 3, what is the mass? ■ D = 10. 49 g/cm 3 ■ M = ? ? ? ■ V = 12. 993 cm 3 ■ 10. 49 = M /12. 993 M = D x V 136. 296 136. 3 g

Practice on Your Own ■ A student finds a rock on the way to school. In the laboratory he determines that the volume of the rock is 22. 7 m. L, and the mass in 39. 943 g. What is the density of the rock? ■ What is the mass of a 350 cm 3 sample of pure silicon with a density of 2. 336 g/cm 3? ■ The density of lead is 11. 342 g/m. L. What would be the volume of a 200. 0 g sample of this metal?

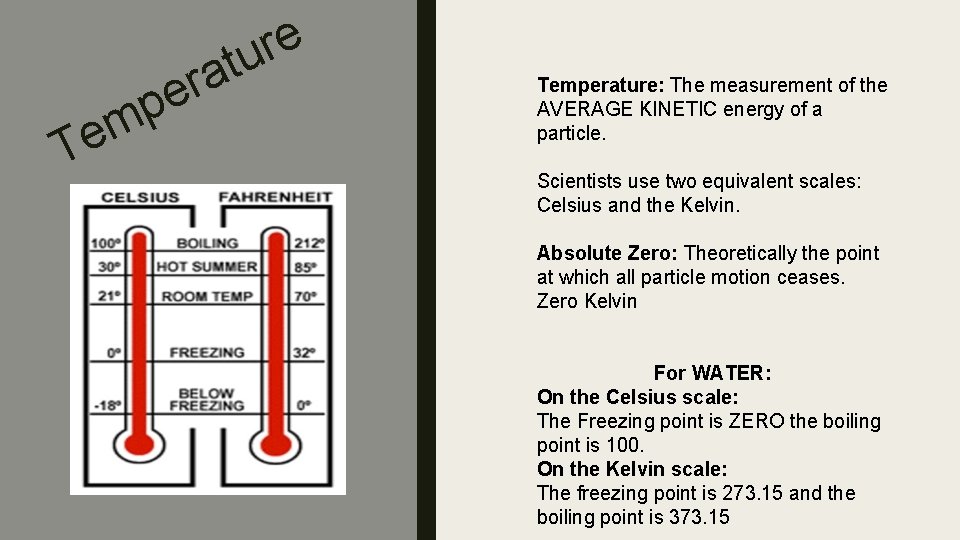
m e T r e p e r u t a Temperature: The measurement of the AVERAGE KINETIC energy of a particle. Scientists use two equivalent scales: Celsius and the Kelvin. Absolute Zero: Theoretically the point at which all particle motion ceases. Zero Kelvin For WATER: On the Celsius scale: The Freezing point is ZERO the boiling point is 100. On the Kelvin scale: The freezing point is 273. 15 and the boiling point is 373. 15

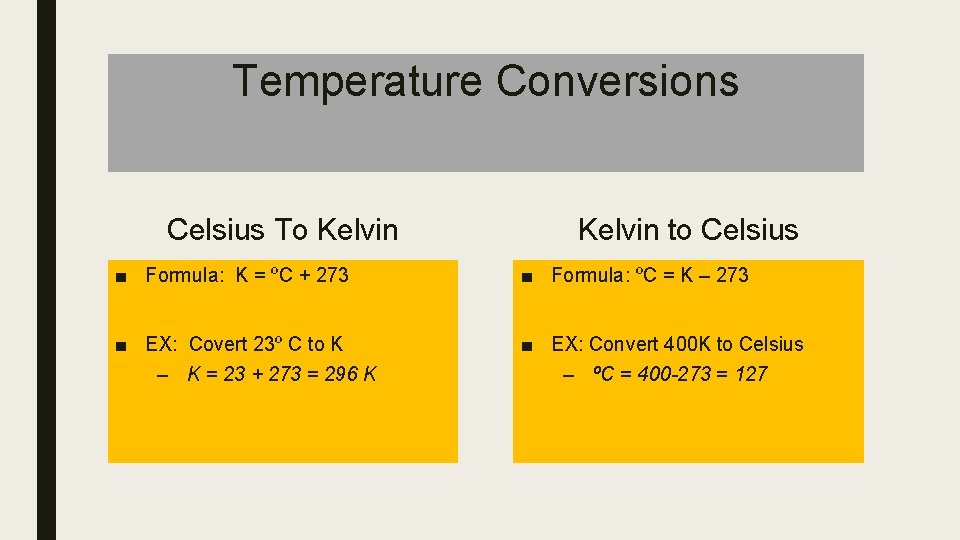
Temperature Conversions Celsius To Kelvin to Celsius ■ Formula: K = ºC + 273 ■ Formula: ºC = K – 273 ■ EX: Covert 23º C to K – K = 23 + 273 = 296 K ■ EX: Convert 400 K to Celsius – ºC = 400 -273 = 127

Practice ■ Convert the Celsius temperatures to Kelvin: – -50ºC – 100ºC – 32ºC ■ Convert the Kelvin temperatures to Celsius: – 150 K – 300 K – 125 K – 25 K