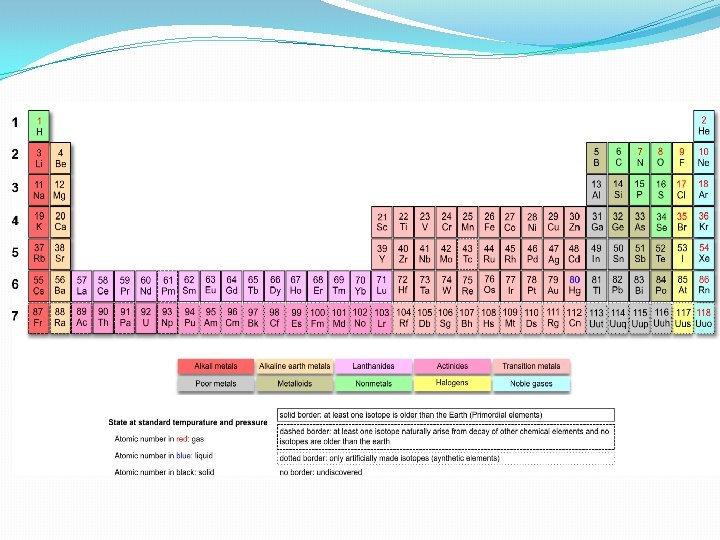
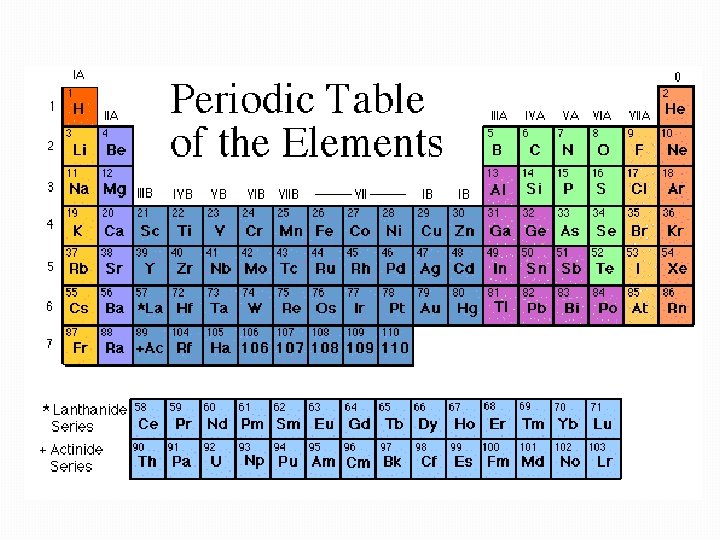
Demystifying the Periodic Table Another possibility Spiral Periodic

Demystifying the Periodic Table
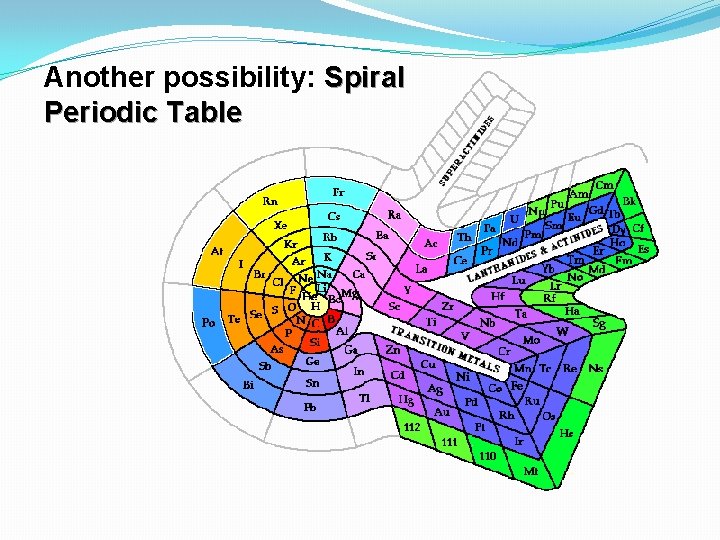
Another possibility: Spiral Periodic Table
The Father of the Periodic Table Dimitri Mendeleev ________was the first scientist to elements notice the relationship between the _____ atomic _______ mass Arranged his periodic table by ____ unknown Said properties of ______elements could be predicted by the properties of elements around the missing element Moseley _____later discovered that the periodic nature of the elements was associated with_____ atomic _____, number not atomic mass
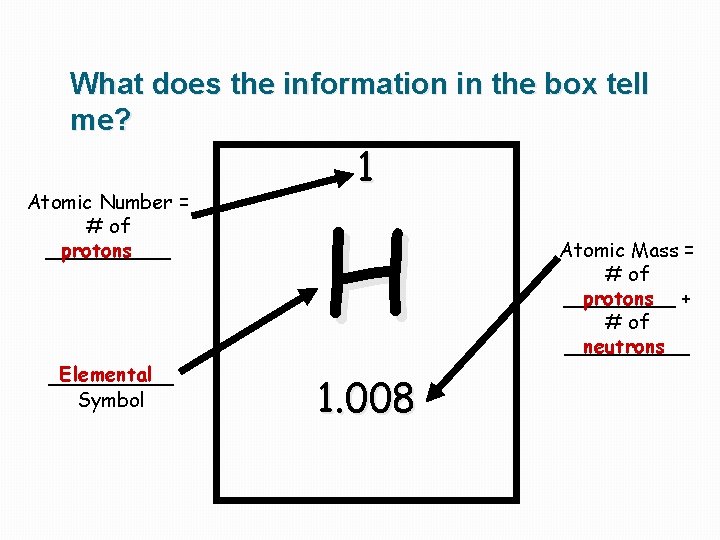
What does the information in the box tell me? 1 Atomic Number = # of _____ protons H Elemental _____ Symbol 1. 008 Atomic Mass = # of _____ protons + # of _____ neutrons
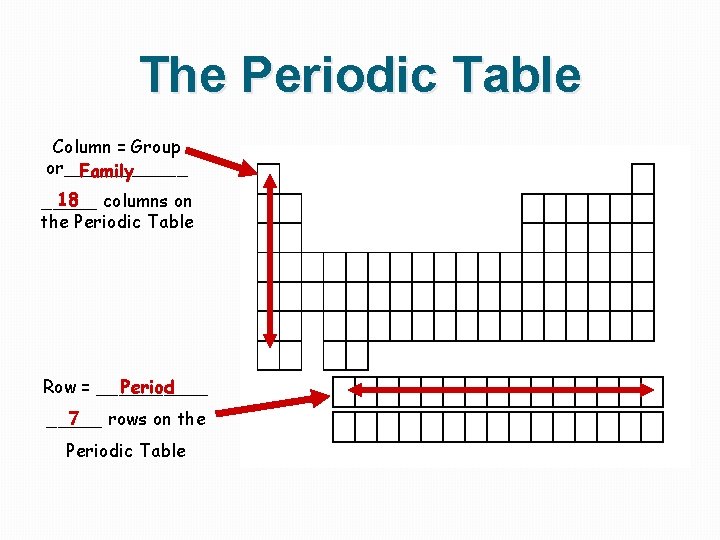
The Periodic Table Column = Group or______ Family 18 columns on _____ the Periodic Table Row = _____ Period 7 _____ rows on the Periodic Table
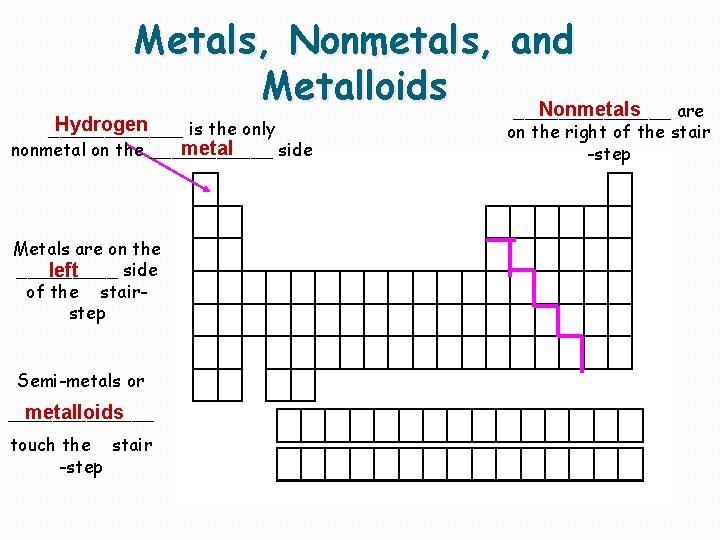
Metals, Nonmetals, and Metalloids Nonmetals _______ are Hydrogen ______ is the only metal nonmetal on the ______ side Metals are on the _____ side left of the stairstep Semi-metals or metalloids _______ touch the stair -step on the right of the stair -step
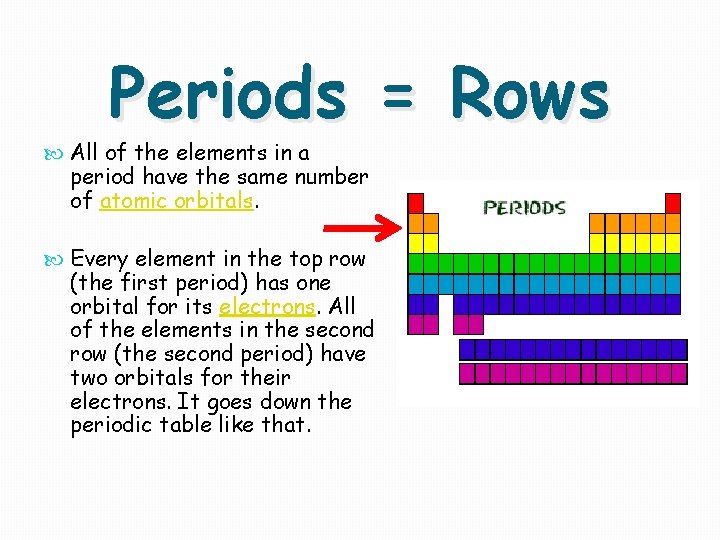
Periods = Rows All of the elements in a period have the same number of atomic orbitals. Every element in the top row (the first period) has one orbital for its electrons. All of the elements in the second row (the second period) have two orbitals for their electrons. It goes down the periodic table like that.
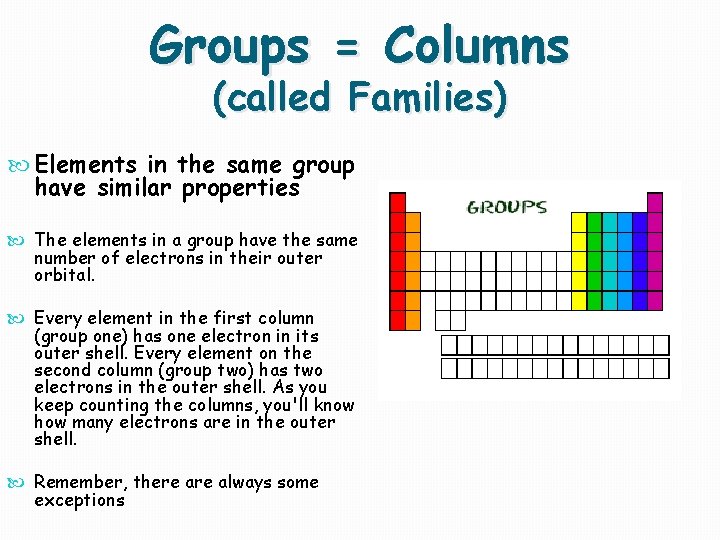
Groups = Columns (called Families) Elements in the same group have similar properties The elements in a group have the same number of electrons in their outer orbital. Every element in the first column (group one) has one electron in its outer shell. Every element on the second column (group two) has two electrons in the outer shell. As you keep counting the columns, you'll know how many electrons are in the outer shell. Remember, there always some exceptions
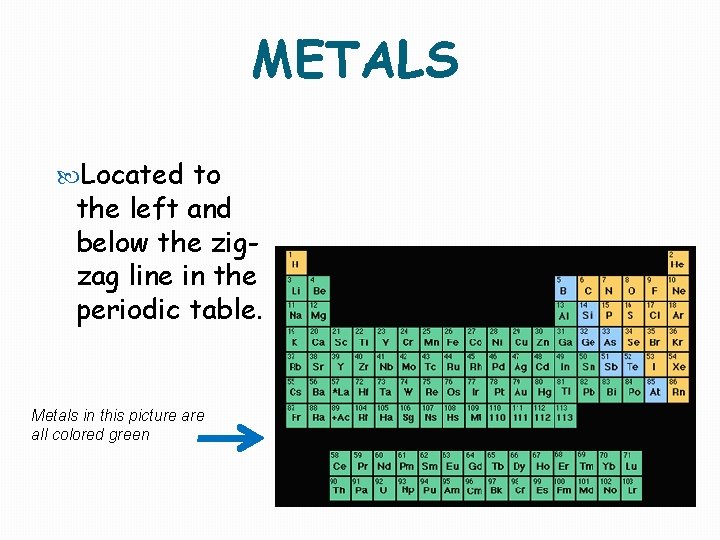
METALS Located to the left and below the zigzag line in the periodic table. Metals in this picture all colored green

Properties of Metals solids Metals are usually _____at room temperature Good conductors of heat and electricity High melting points and boiling points (except Hg) High densities (except some group 1 metals) luster Metals have ______(are shiny) and reflect light

Properties of Metals Malleable ______ - can be bent, pounded into sheets Ductile – can be stretched into wire ____ without breaking
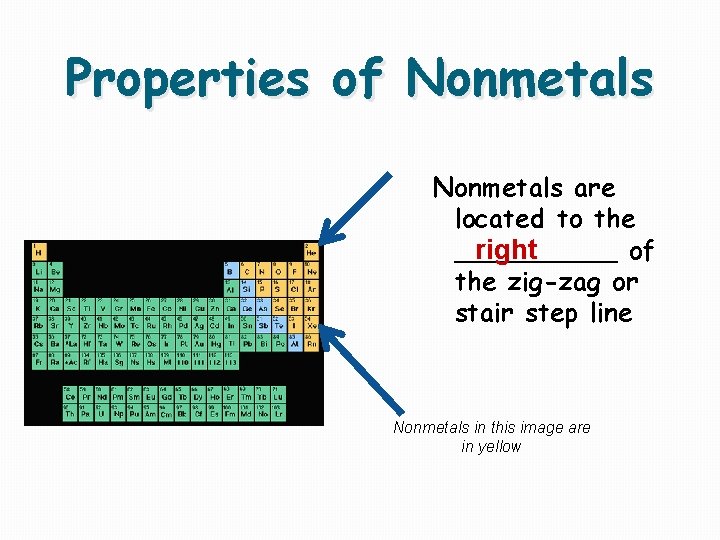
Properties of Nonmetals are located to the _____ of right the zig-zag or stair step line Nonmetals in this image are in yellow
Properties of Nonmetals The nonmetals exist in all three of the states of matter at room temperature: _____ gases liquids (such as oxygen), _____(such as bromine), solids and ______ (such as carbon).
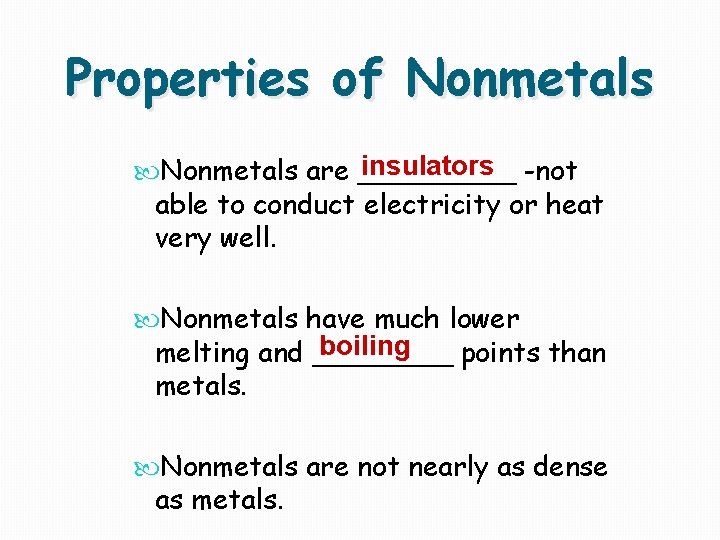
Properties of Nonmetals insulators -not Nonmetals are _____ able to conduct electricity or heat very well. Nonmetals have much lower boiling melting and ____ points than metals. Nonmetals are not nearly as dense as metals.
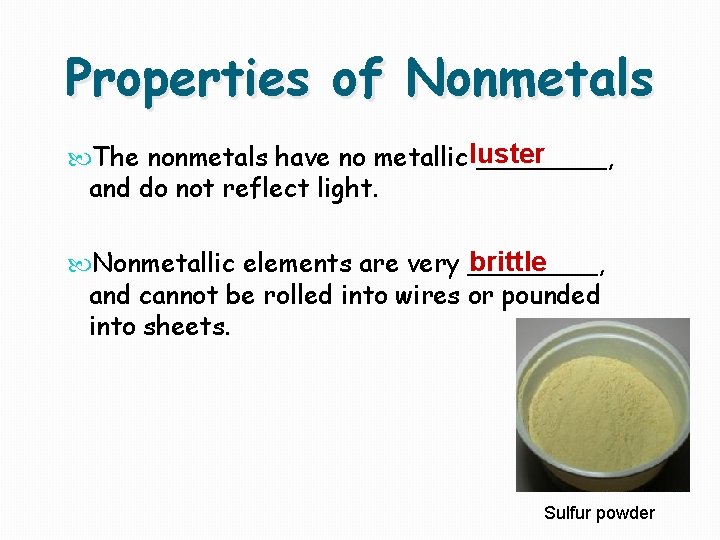
Properties of Nonmetals The nonmetals have no metallic luster ____, and do not reflect light. brittle Nonmetallic elements are very ____, and cannot be rolled into wires or pounded into sheets. Sulfur powder
Hyrdrogen OH, BOTHER!! The Group 1 Nonmetal - the one element located on the “metal” side of the periodic table, that is not a metal. Most of the hydrogen found in the world is found in water.
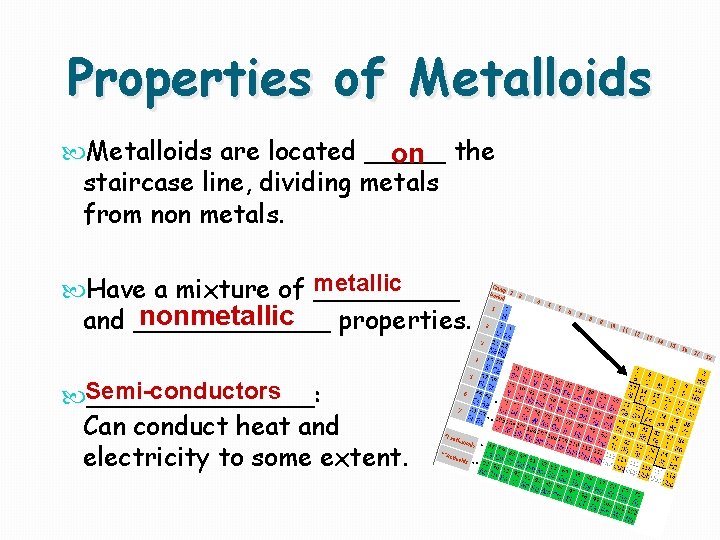
Properties of Metalloids are located _____ on the staircase line, dividing metals from non metals. metallic Have a mixture of _____ nonmetallic __ properties. and ______ Semi-conductors _______: Can conduct heat and electricity to some extent.
- Slides: 19