Demos n n n n n Shuttle Tile


















































- Slides: 78

Demos n n n n n Shuttle Tile and Oven Mohair fuzz Candle Fire Syringe Deck of Cards Pasco Cylinder and Temp Sensor Bicycle Tire and Digital Thermometer Steam Engine and generator + bulb Stirling Engine n DI Water , Ethanol

Thermodynamics, Heat Energy, and how we benefit from it.

Outline n n What is Thermodynamics What is Heat and temperature n n Definition What makes Heat flow (heat transfer) Specific Heat How heat flows n n n Conduction Convection Radiation n Laws of Thermodynamics and Entropy Doing work on a Fluid n n Fire Piston - before the invention of the Match Head Some common thermodynamic cycles n Otto Cycle and other heat engines n n n Electromagnetic Spectrum Combustion Process Stirling Refrigeration and heat pumps Why 100% efficiency is theoretically impossible (the Heat Tax) Common engines n n n Steam Engine Diesel Engine Electric Engine

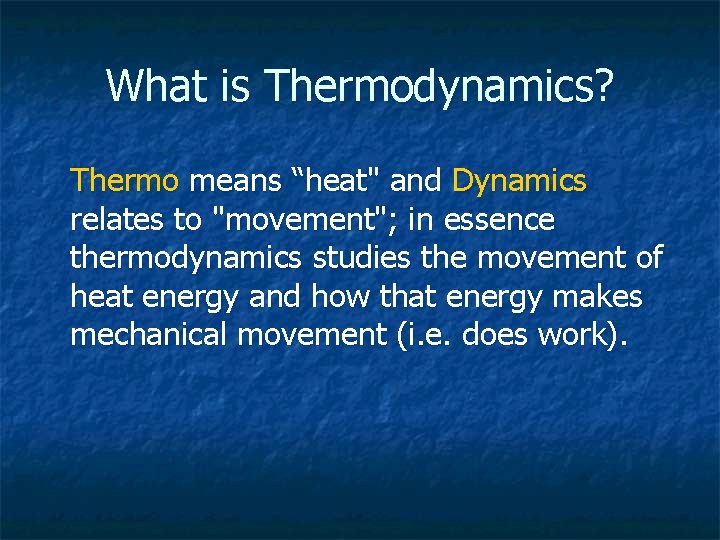
What is Thermodynamics? Thermo means “heat" and Dynamics relates to "movement"; in essence thermodynamics studies the movement of heat energy and how that energy makes mechanical movement (i. e. does work).

Thermodynamics is a science about the effects of changes in temperature, pressure, and volume and how these changes effect a physical system. (e. g. a car engine, an air conditioner)

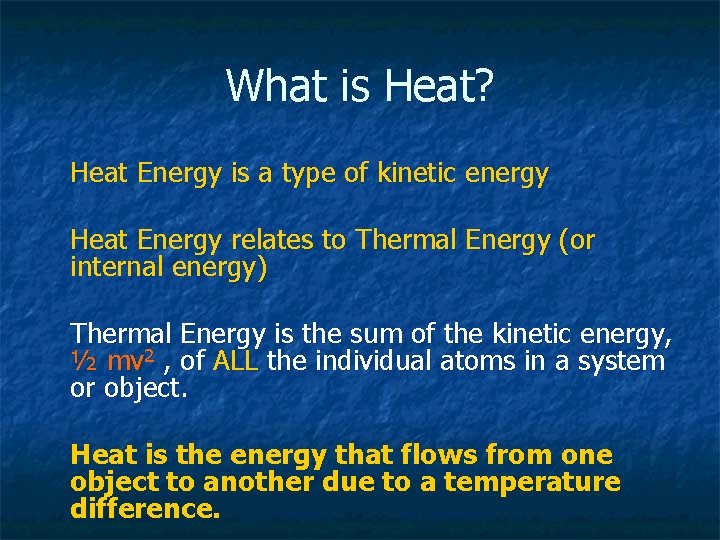
What is Heat? Heat Energy is a type of kinetic energy Heat Energy relates to Thermal Energy (or internal energy) Thermal Energy is the sum of the kinetic energy, ½ mv 2 , of ALL the individual atoms in a system or object. Heat is the energy that flows from one object to another due to a temperature difference.

When Energy flows from a hot object to a cold object, the energy is called Heat http: //hop. concord. org/htu. concepts. flow. html

Before 1800, Heat was thought to be an invisible fluid that flowed between objects n n n Objects were thought to contain fixed quantities of heat. Benjamin Thompson observed that canons bored with dull tools became very hot while those bored with sharp tools did not get as hot. The heat generated had nothing to do with the size of the canon. Thompson suggested that heat came from friction (or mechanical energy). http: //honolulu. hawaii. edu

James Joule tests the predictions: James Joule’s experiment proved that heat was a form of energy. In this experiment the kinetic energy of the paddle is transferred to thermal energy in the water, as measured with a sensitive thermometer. http: //www. geocities. com/bioelectrochemistry/joule. htm

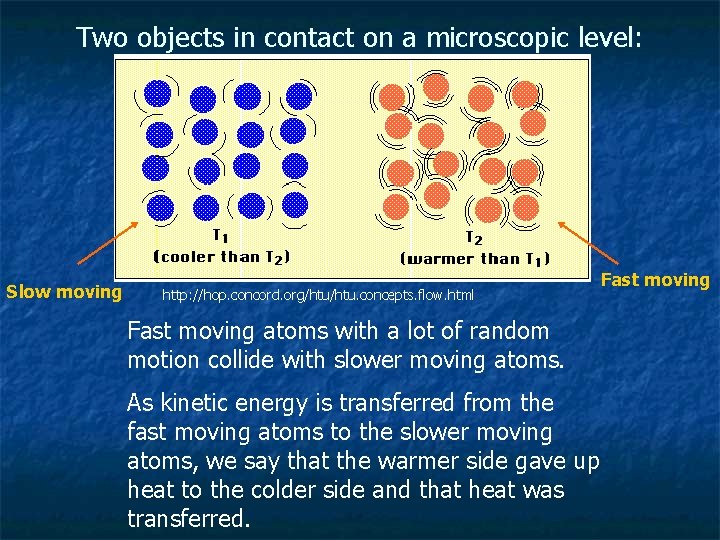
Two objects in contact on a microscopic level: Slow moving http: //hop. concord. org/htu. concepts. flow. html Fast moving atoms with a lot of random motion collide with slower moving atoms. As kinetic energy is transferred from the fast moving atoms to the slower moving atoms, we say that the warmer side gave up heat to the colder side and that heat was transferred. Fast moving

What is Temperature? Temperature is a measurement of the average thermal energy of the particles in a substance. Heat flows due to temperature differences. No heat is transferred between two objects that are at the same temperature (i. e. in thermal equilibrium). A cup of boiling water is at the same temperature as a gallon of boiling water, but the gallon of boiling water has more thermal energy than the cup.

Which object has higher thermal energy? http: //picasaweb. google. com/peppermint. patti 1960

Heat Capacity n n n Heat Capacity of an object is the required energy needed to raise the object’s temperature by one degree. A large quantity of matter has a larger heat capacity than something smaller Our oceans and atmosphere have large heat capacities due to their large sizes.

Specific Heat The measure of the heat energy required to increase the temperature of a unit quantity of a substance by one degree. Copper Dry Air Humid Air Water Concrete Sand 0. 385 Joule/gr o. C 1. 0035 Joule/gr o. C 1. 0102 Joule/gr o. C 4. 1813 Joule/gr o. C 0. 88 Joule/gr o. C 0. 42 Joule/gr o. C Second highest specific heat, next to Ammonia

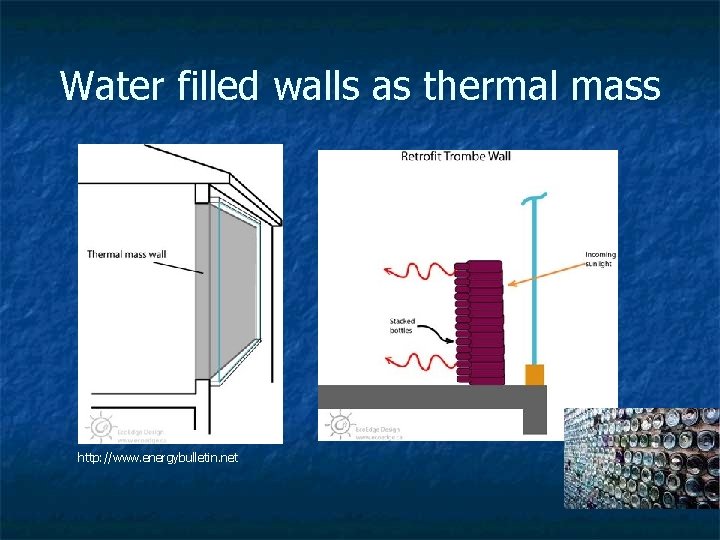
Specific Heat of Water n n Very high Earth’s ocean store vast amounts of thermal energy – these large heat reservoirs regulate the earth’s temperature. Unfrozen lakes moderate surrounding climate Water filled walls make good thermal mass

Water filled walls as thermal mass http: //www. energybulletin. net

Using plant material as solar mass Mc. Gill University, Montreal – Solar Decathlon 2007 http: //www. solardecathlon. org/

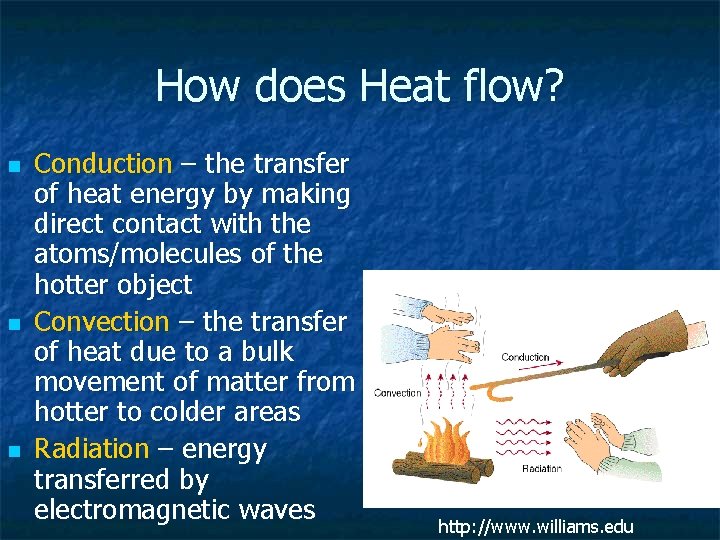
How does Heat flow? n n n Conduction – the transfer of heat energy by making direct contact with the atoms/molecules of the hotter object Convection – the transfer of heat due to a bulk movement of matter from hotter to colder areas Radiation – energy transferred by electromagnetic waves http: //www. williams. edu

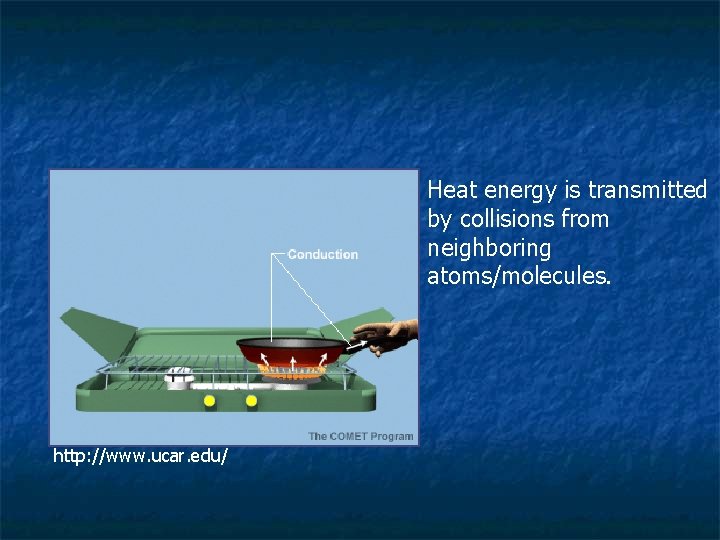
Conduction n When two objects are in direct contact, particles in the hotter object are moving faster and will collide with slower moving objects in the colder object. When this happens, heat flows. Energy is transferred from the hot object to the cold object.

Touch the wood table and then touch the metal legs of the table… Both Objects are at the same temperature, but the metal feels colder, why? You are at a higher temperature than any nonliving object in the room, therefore heat is transferred from your body to both the wood and the metal. The metal conducts heat better than the wood because there a lot of free electrons in metals, therefore mobile electrons take heat from your hand faster than wood.

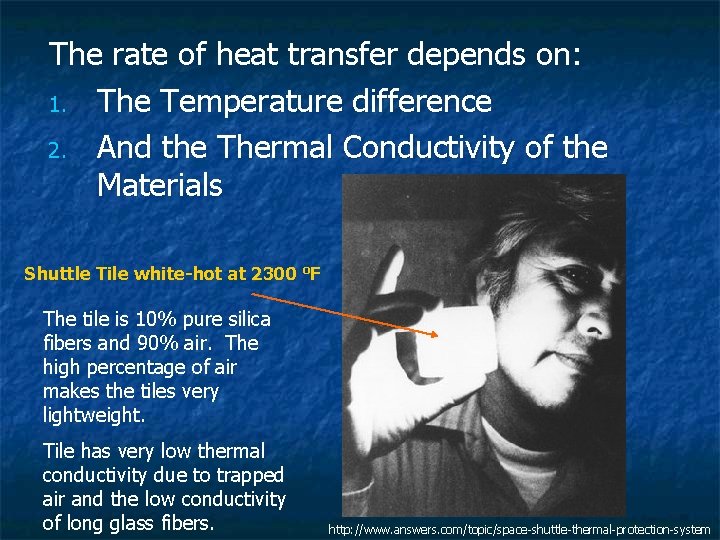
The rate of heat transfer depends on: 1. The Temperature difference 2. And the Thermal Conductivity of the Materials Shuttle Tile white-hot at 2300 o. F The tile is 10% pure silica fibers and 90% air. The high percentage of air makes the tiles very lightweight. Tile has very low thermal conductivity due to trapped air and the low conductivity of long glass fibers. http: //www. answers. com/topic/space-shuttle-thermal-protection-system

Is air a good thermal insulator? n n n Thermal Insulation is the method of preventing heat from entering or escaping from a container. Stagnant air is a good thermal insulator Coats, feathers, fur, hair, fiberglass insulation, & straw bales all trap tiny pockets of air. The ocean of air over your head helps keep the earth cool during the day and warm during the night. Air has high specific heat.

Heat energy is transmitted by collisions from neighboring atoms/molecules. http: //www. ucar. edu/

More examples of Conduction www. backpackgeartest. org www. broadys. co. nz

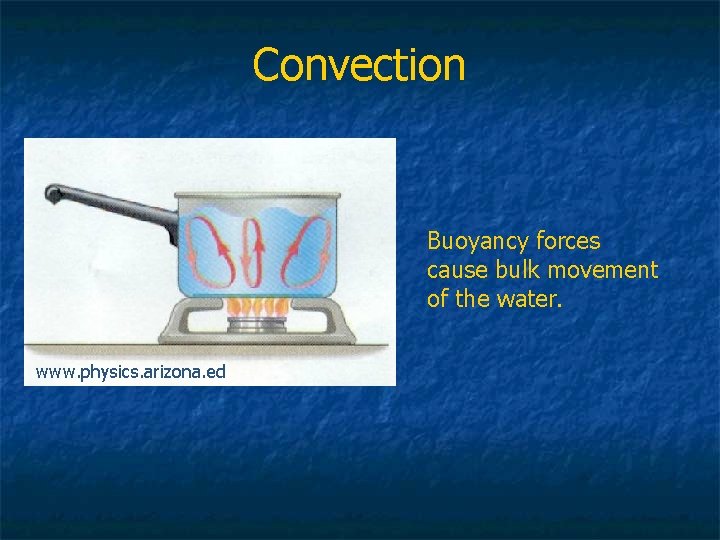
Convection Buoyancy forces cause bulk movement of the water. www. physics. arizona. ed

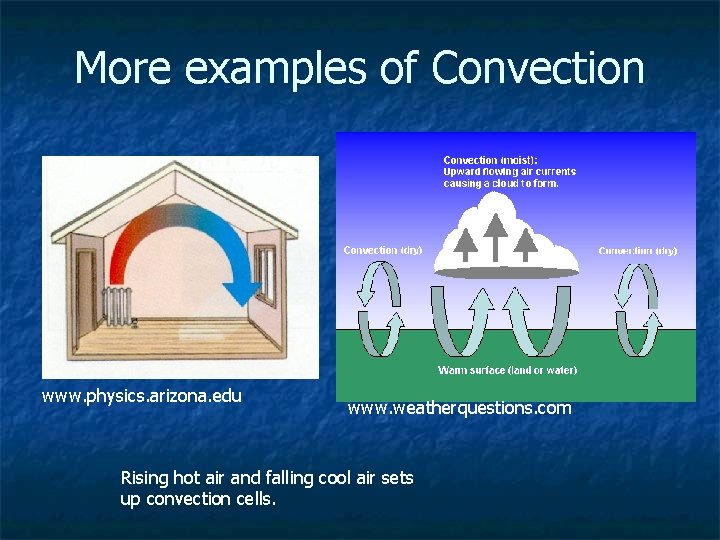
More examples of Convection www. physics. arizona. edu www. weatherquestions. com Rising hot air and falling cool air sets up convection cells.

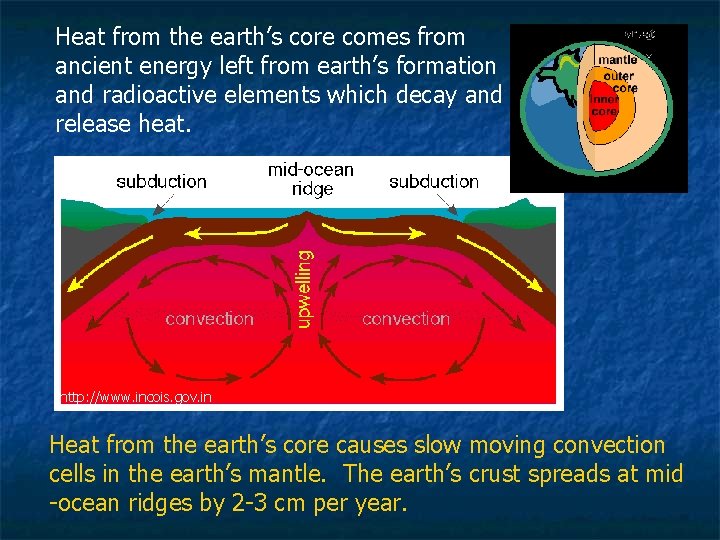
Heat from the earth’s core comes from ancient energy left from earth’s formation and radioactive elements which decay and release heat. http: //www. incois. gov. in Heat from the earth’s core causes slow moving convection cells in the earth’s mantle. The earth’s crust spreads at mid -ocean ridges by 2 -3 cm per year.

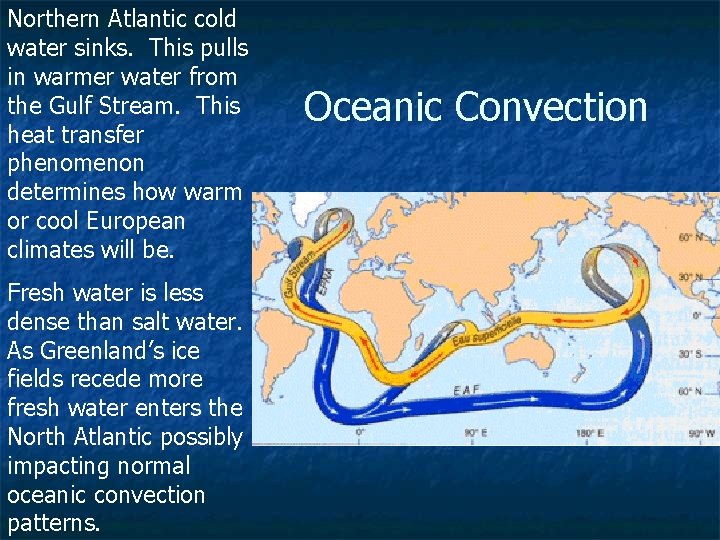
Northern Atlantic cold water sinks. This pulls in warmer water from the Gulf Stream. This heat transfer phenomenon determines how warm or cool European climates will be. Fresh water is less dense than salt water. As Greenland’s ice fields recede more fresh water enters the North Atlantic possibly impacting normal oceanic convection patterns. Oceanic Convection

Forced Convection n n Forced Convection is not due to the natural forces of buoyancy induced by heating. Instead, there is a external force that causes the fluid to convect, such as a fan or a pump.

Convection Ovens A fan circulates the air so hot air is not trapped at the top of the oven. More cookies can be baked at one time and all will cook at the same rate.

Ceiling Fans www. sleekhome. com In both hot and cold weather, ceiling fans are useful for circulating air to force convection. Rooms with high ceilings are a problem during the winter as the hot air rises and moves away from the floor area.

Heat Transfer from Radiation n All matter that has thermal energy will emit infrared electromagnetic radiation. We can feel this when we put our hands close to a fire. This type of heat transfer requires no medium. Electromagnetic radiation travels at the speed of light through a vacuum. http: //www. newt. com http: //www. charlesandhudson. com

Infrared Radiation All objects with thermal energy emit Infrared Radiation (even ice) Infrared radiation is invisible to our eyes but we can feel it as heat Nasa. gov


The Sun’s energy is transferred to earth by electromagnetic waves n n n Visible Light Infrared radiation Ultraviolet (UV) http: //www. foxnews. com

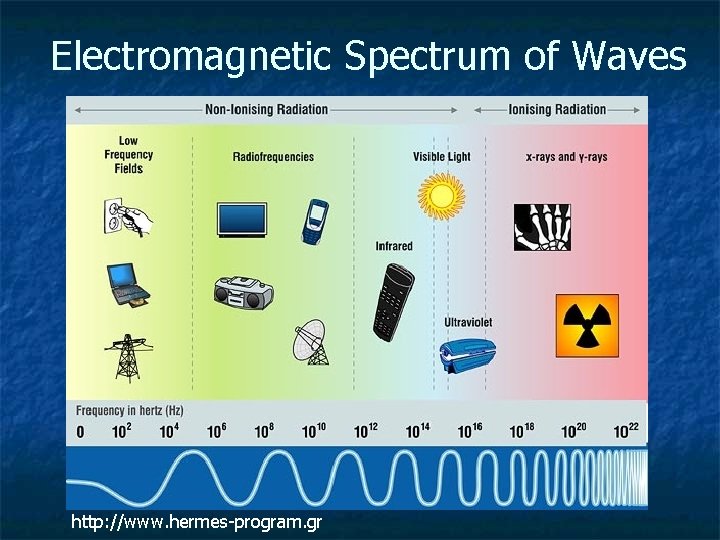
Electromagnetic Spectrum of Waves http: //www. hermes-program. gr

n n Ionizing radiation causes matter to ionize (can rip an electron off an atom) Ionizing radiation carries more energy than those waves with larger wavelengths. The sun’s UV waves are those responsible for burning and skin cancer. Infrared is non-ionizing radiation. Non-ionizing radiation is everywhere and is considered to not be harmful.

Laws of Thermodynamics Zeroth Law: If two objects are in thermal equilibrium with a third object, then they are also in thermal equilibrium with each other. Thermal equilibrium means an objects temperature, pressure, and volume are not changing.

http: //www. cafemakers. com A cooling cup of coffee is NOT in thermal equilibrium with the room.

If two cups of coffee are at thermal equilibrium with the room, then the two cups are in thermal equilibrium with each other. The two cups of coffee have the same temperature. /www. wentapottery. com If the two cups are put in contact with each other no heat will flow.

First Law of Thermodynamics: (The good news!) Energy is Conserved. Energy can not be destroyed. In an isolated system, the total energy stays the same. Energy can be converted from one form to another. Thermal Energy can be converted into another form of energy!

What is Entropy? Entropy = total disorder of an object/system Disorder is the sum of thermal energy plus the physical disorder. Entropy always increases with time!

Examples of increasing entropy Wikipedia. com www. wiley. com Playing “ 52 pick up”

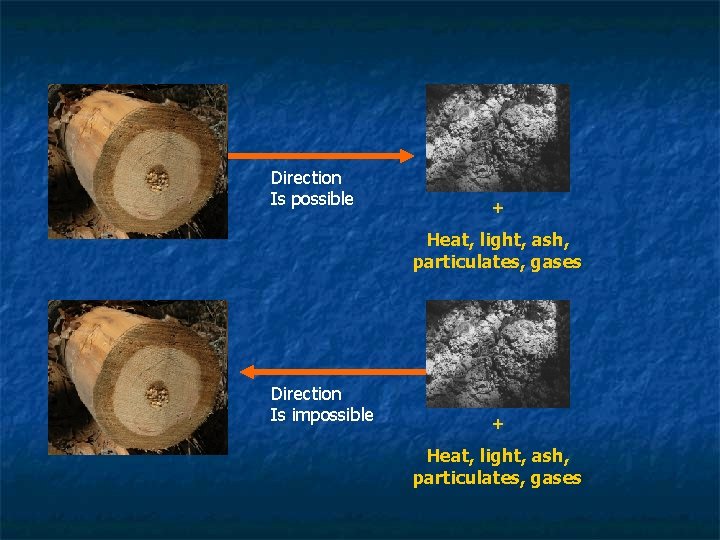
Direction Is possible + Heat, light, ash, particulates, gases Direction Is impossible + Heat, light, ash, particulates, gases

Energy flows in one direction – towards a more disordered state

The Second Law of Thermodynamics: (The bad news!) An isolated system gets more disordered with time. Entropy always increases with time.

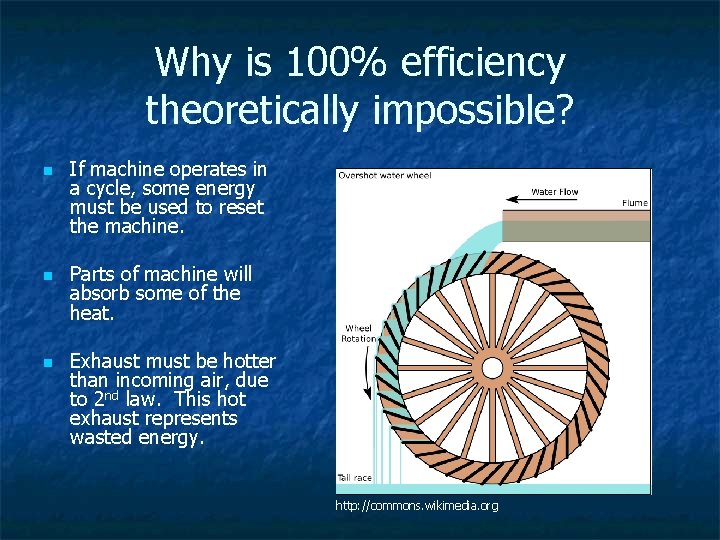
What does this mean to us? n n It is impossible to construct an engine that converts all its thermal energy into useful work. The exhaust must be hotter than the incoming air. 100% efficiency is impossible –there must be some unusable energy because entropy must increase. We’re going to get old and die The house is going to need cleaning again!

Why is 100% efficiency theoretically impossible? n n n If machine operates in a cycle, some energy must be used to reset the machine. Parts of machine will absorb some of the heat. Exhaust must be hotter than incoming air, due to 2 nd law. This hot exhaust represents wasted energy. http: //commons. wikimedia. org

Doing work on a Fluid When a fluid is compressed, work is done on the fluid. This work/energy is converted into thermal energy within the fluid. Each molecule has more kinetic energy so the temperature of the fluid increases.

As air is rapidly compressed, it can reach 400 -500 degrees, allowing tinder to ignite. Fire Piston www. grannysstore. com The compressed air is the heat source as well as the oxygen needed to ignite the tinder. Fire piston are thought to be prehistoric fire starting devices, used in South East Asia and South Pacific. For more info see: http: //en. wikipedia. org/wiki/Fire_piston

The modern match evolved during the 1800’s. Prior to 1900, fires had to be maintained or started by creating heat through friction. Wikipedia. com The Bow and Drill used by Native Americans Many people used flintlock guns. http: //wildwoodsurvival. com

But fluids can do work on surroundings n n A compressed gas will experience an increase in pressure (as well as an increase in temperature if compression is fast). When a pressurized gas expands it’s thermal energy decreases because it is doing work (it is exerting forces as it expands). n Ex. Air escaping from a bicycle tire feels cold.

Heat Engines A cycling machine/engine that converts thermal energy into mechanical energy (also known as work) Examples: 4 -stroke engine (OTTO Cycle) Steam Engines Stirling Engine

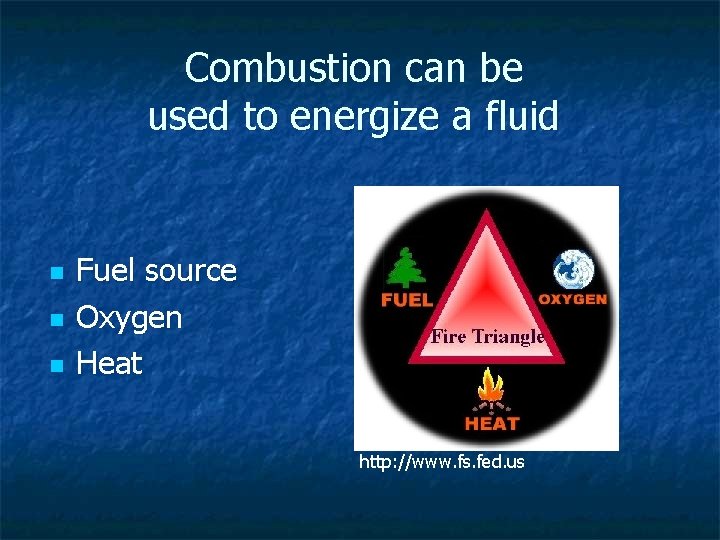
Combustion can be used to energize a fluid n n n Fuel source Oxygen Heat http: //www. fs. fed. us

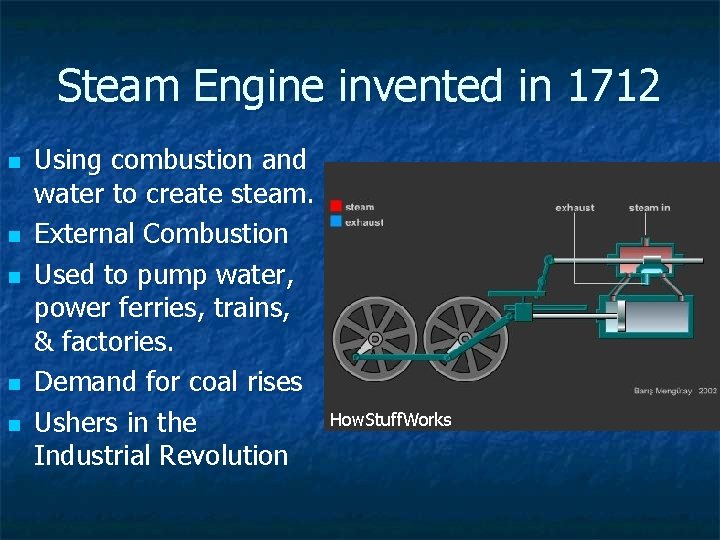
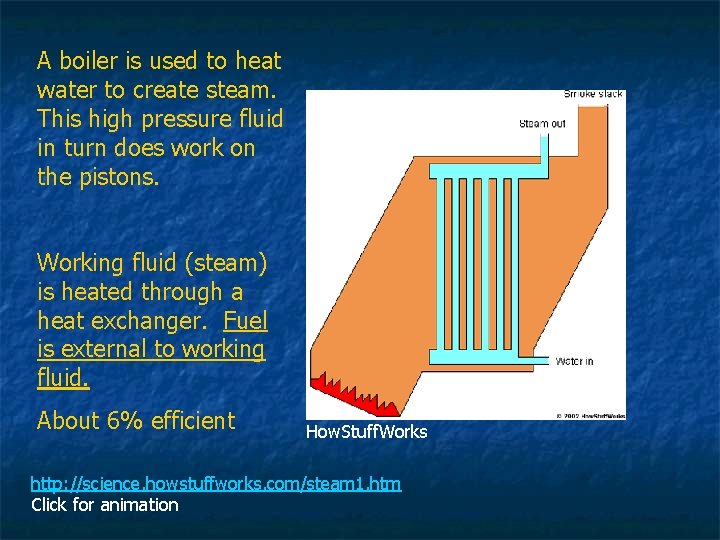
Steam Engine invented in 1712 n n n Using combustion and water to create steam. External Combustion Used to pump water, power ferries, trains, & factories. Demand for coal rises Ushers in the Industrial Revolution How. Stuff. Works

A boiler is used to heat water to create steam. This high pressure fluid in turn does work on the pistons. Working fluid (steam) is heated through a heat exchanger. Fuel is external to working fluid. About 6% efficient How. Stuff. Works http: //science. howstuffworks. com/steam 1. htm Click for animation

Coal and Steam Powered Factory Briggs and Stratton Website

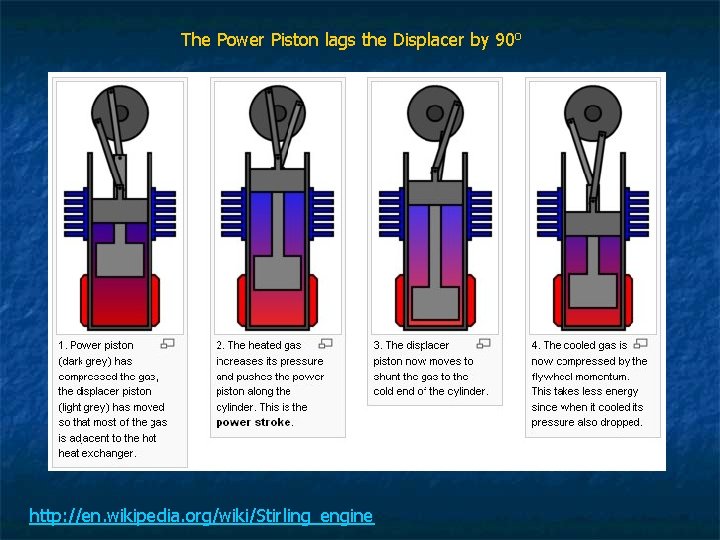
Stirling Engine - 1816 n n Closed Cycle – working fluid is contained within the system Highest efficiency possible Higher capital costs Heat Source and Heat Sink needed

The Power Piston lags the Displacer by 90 o http: //en. wikipedia. org/wiki/Stirling_engine

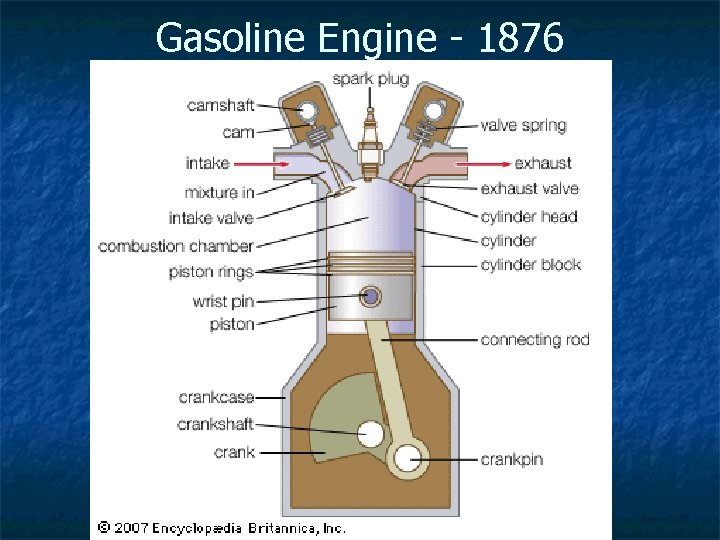
Gasoline Engine - 1876

Gasoline Engines n n Internal Combustion – burning takes place inside the engine Based on a four-stroke combustion cycle called the Otto Cycle: n n Intake Compression Combustion Exhaust http: //auto. howstuffworks. com/engine 1. htm Click for animation

Diesel Engine - 1876 Diesel engines do not have spark plugs because similar to the fire piston, the compression of the gas and air mixture is great enough to automatically ignite the fuel. Gas is injected into cylinder after air is compressed. This allows for greater compression, and higher efficiency. http: //auto. howstuffworks. com/diesel 1. htm Click for animation

Bio. Diesel n n n Made from plant or animal oils Chemically treated so that Bio. Diesel won’t solidify at low temperatures or clog fuel lines Very simply chemistry Make Magazine Vol#3

Efficiency of Engines n n IC Engine is only 20 -30% efficient Diesel is more efficient due to the greater compression rate and ability to extract more work out of the fuel

Why 100% efficiency is impossible? n n At least some of the energy must be passed on to heat a low-temperature energy sink This is due to the 2 nd Law of Thermodynamics – Entropy must increase! Engine needs to be reset. Engine parts will absorb some of the heat energy.

Early cars employed three technologies 1. 2. 3. Steam powered Electric battery powered Gasoline and Diesel powered Stanley Steam Car 1912

Steam Cars n n n Heavy Slow to heat up and start Required carrying both fuel and water http: //www. steamcar. net/my-85. html

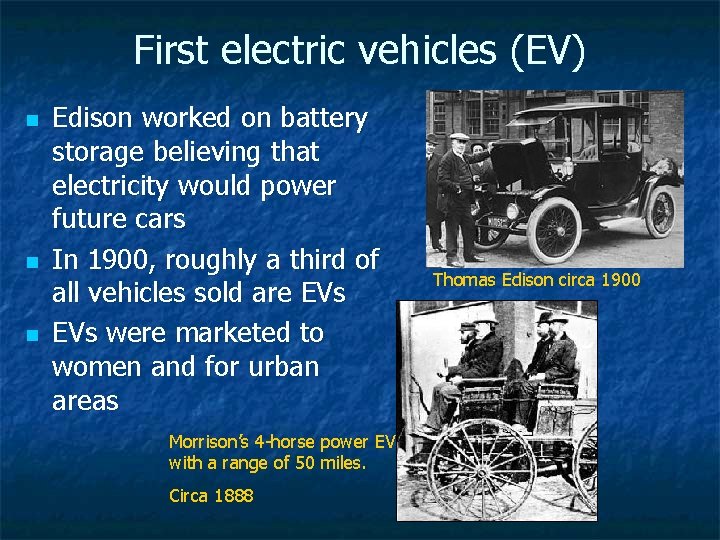
First electric vehicles (EV) n n n Edison worked on battery storage believing that electricity would power future cars In 1900, roughly a third of all vehicles sold are EVs were marketed to women and for urban areas Morrison’s 4 -horse power EV with a range of 50 miles. Circa 1888 Thomas Edison circa 1900

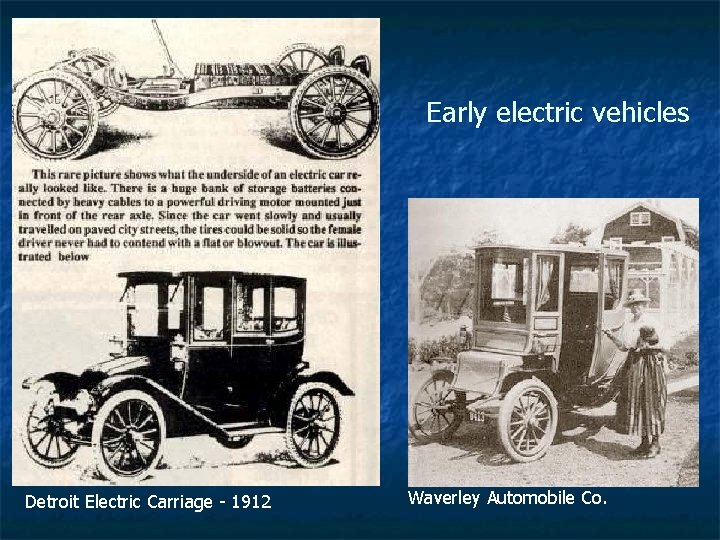
Early electric vehicles Detroit Electric Carriage - 1912 Waverley Automobile Co.

Early Gas powered cars Karl Benz was the first to commercialize a gas powered motorwagon in 1885

Why did EVs and Steamers fade away? n n n Gasoline and Diesel have high energy densities Oil found in Texas Greatest need for cars and trucks was in rural areas, therefore long range was needed. Steamers too heavy on unpaved roads Gas powered cars started quickly Henry Ford perfected the assembly-line, making his cars the most affordable

Why was gasoline the chosen fuel source for the automobile? n n n Gasoline has 1000 X the energy as an equal weight of batteries. Gasoline has 4. 5 X more energy per gallon than liquid hydrogen. Gasoline has 2 X the energy of coal for the same weight Gas has slightly less energy per volume as veggie oil Gasoline combines with Oxygen when it burns. The Oxygen is free and does not have to be carried.

Huntington Beach 1928 Beaumont, Texas on Spindletop Hill

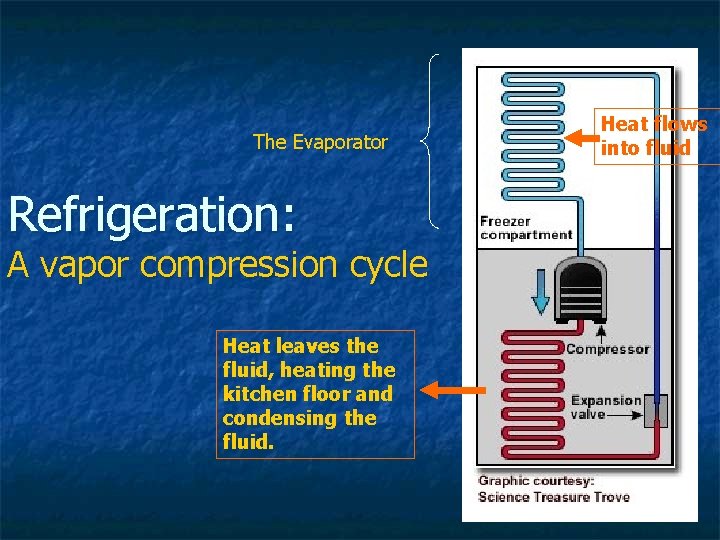
The Evaporator Refrigeration: A vapor compression cycle Heat leaves the fluid, heating the kitchen floor and condensing the fluid. Heat flows into fluid

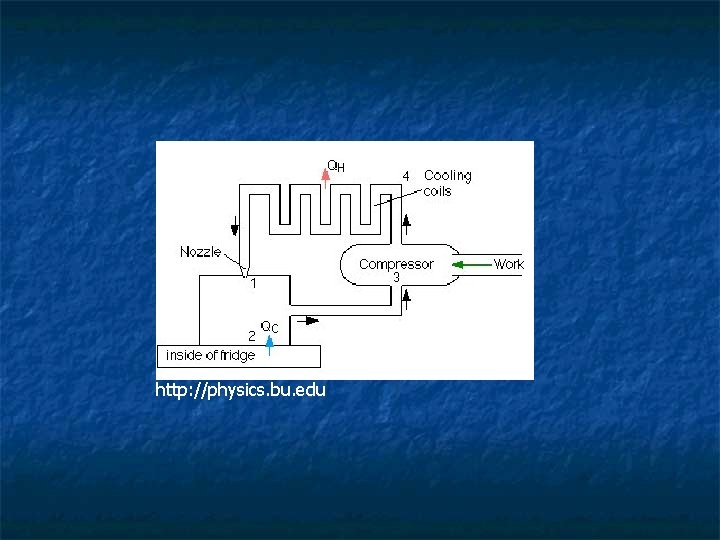
http: //physics. bu. edu

The compressor on the bottom compresses the working fluid raising its temperature. In the condenser coils, heat leaves the fluid and enters the room. This condenses the fluid into a liquid. When allowed to expand, the temperature of the liquid drops dramatically. This cold fluid absorbs heat from the inside of the refrigerator, causing the fluid to evaporate and turn back into a gas. http: //www. lpappliances. com

Which law of Thermodynamics does the following video demonstrate? http: //www. youtube. com/watch? v=U 82 e Wpt. Fx. Ss

Internal Combustion vs. External Combustion – the fluid doing the work (working fluid) is heated externally. Internal Combustion – the fluid doing the work is heated by burning a fuel internally inside a cylinder pushing down on a piston n Pros of IC n electric starter, so easier and quick to start up Cons of IC n By-products of combustion in exhaust gases n Major Plus Pros of EC n Fuel can be anything Cons of EC n Slow to start n Heat exchanger needed n If steam is the working fluid: n Boiler needed n Water freezes at low temperatures