Demos for Free Energy that is Chemical Demonstrations






- Slides: 37

Demos for Free (Energy, that is. . ) Chemical Demonstrations Explained with Graphs of Free Energy vs. Temperature Patrick Riley, Robert Hanson, Paul Fischer and Jeff Schwinefus Department of Chemistry, St. Olaf College Department of Chemistry, Macalaster College July 19, 2004

“Demo Day” A skit is performed to demonstrate recrystallization. Can we recover our “contaminated” goods?

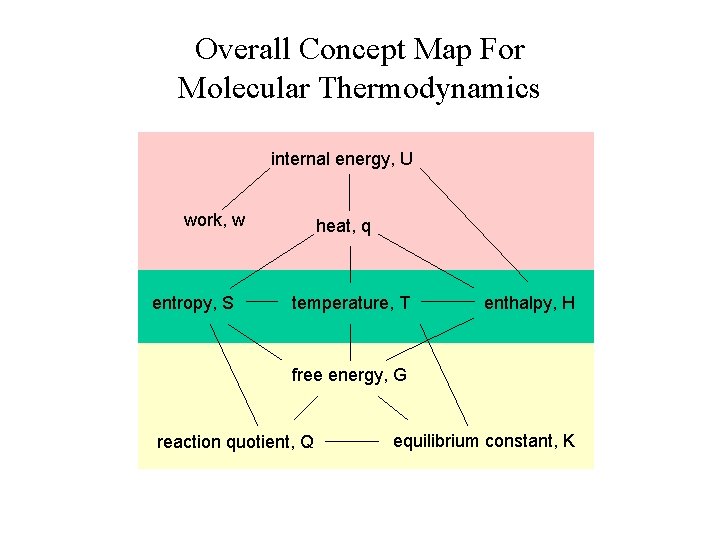
Overall Concept Map For Molecular Thermodynamics internal energy, U work, w entropy, S heat, q temperature, T enthalpy, H free energy, G reaction quotient, Q equilibrium constant, K

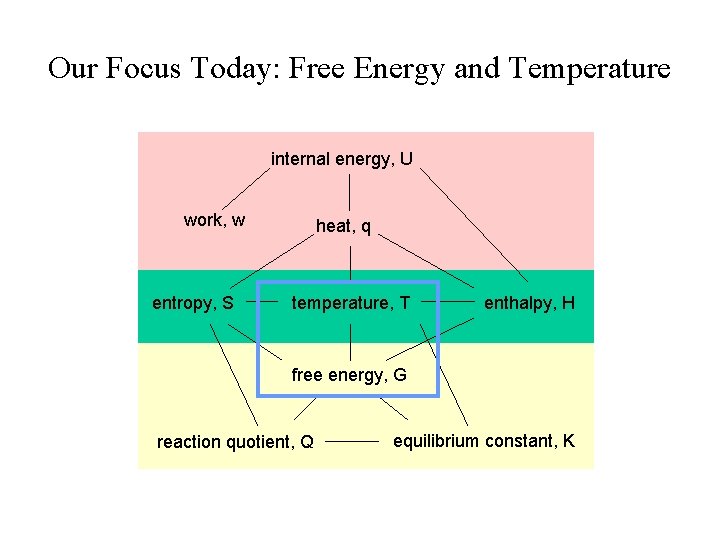
Our Focus Today: Free Energy and Temperature internal energy, U work, w entropy, S heat, q temperature, T enthalpy, H free energy, G reaction quotient, Q equilibrium constant, K

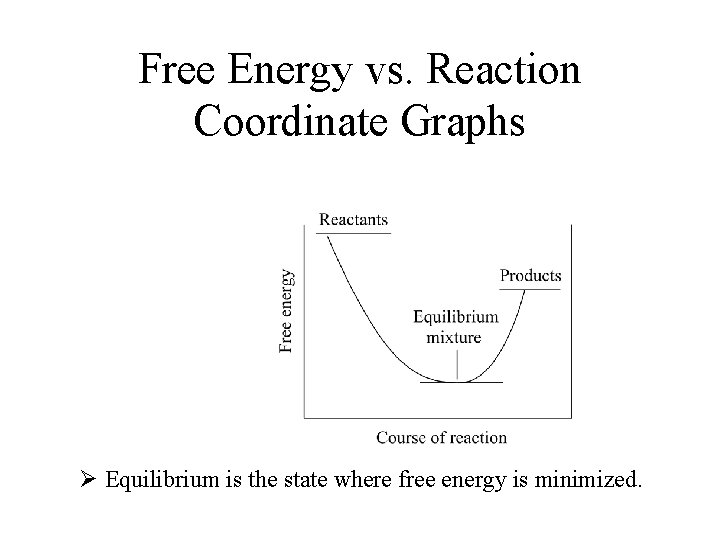
Free Energy vs. Reaction Coordinate Graphs Ø Equilibrium is the state where free energy is minimized.

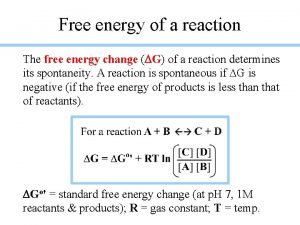
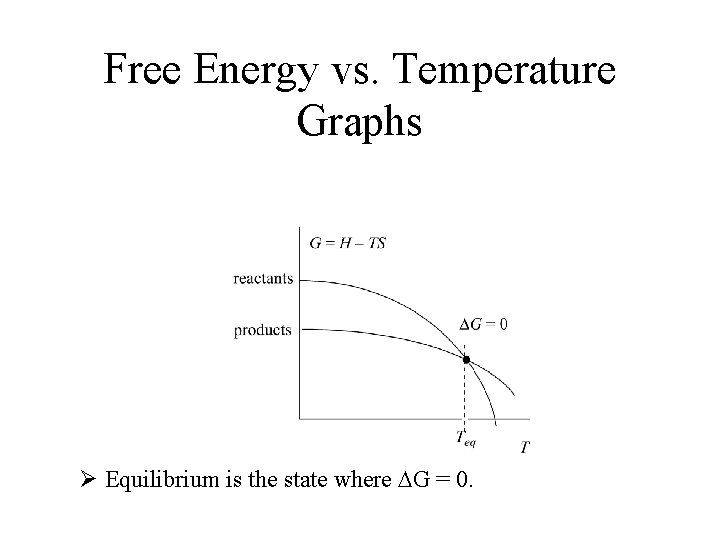
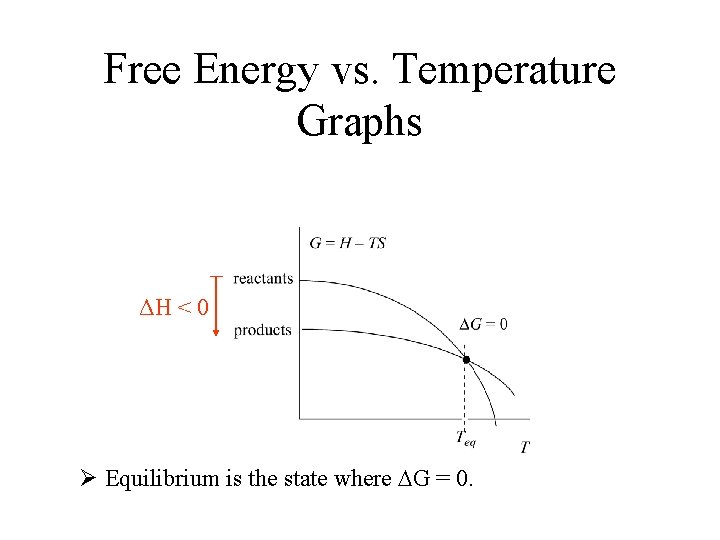
Free Energy vs. Temperature Graphs Ø Equilibrium is the state where DG = 0.

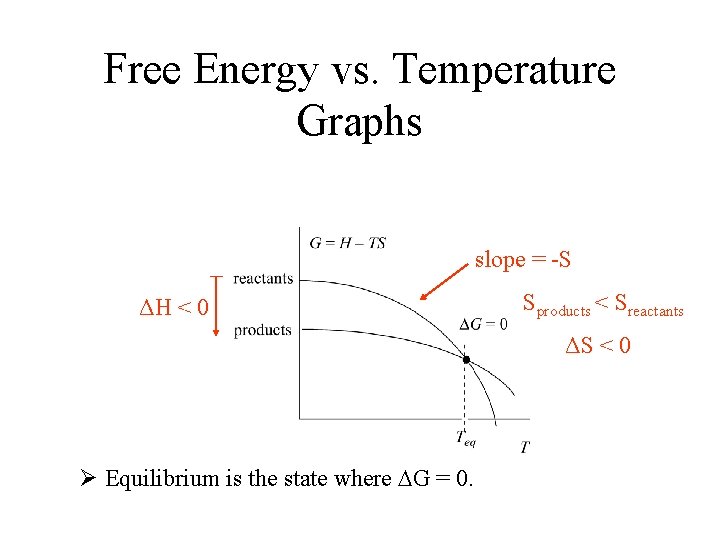
Free Energy vs. Temperature Graphs ΔH < 0 Ø Equilibrium is the state where DG = 0.

Free Energy vs. Temperature Graphs slope = -S ΔH < 0 Sproducts < Sreactants ΔS < 0 Ø Equilibrium is the state where DG = 0.

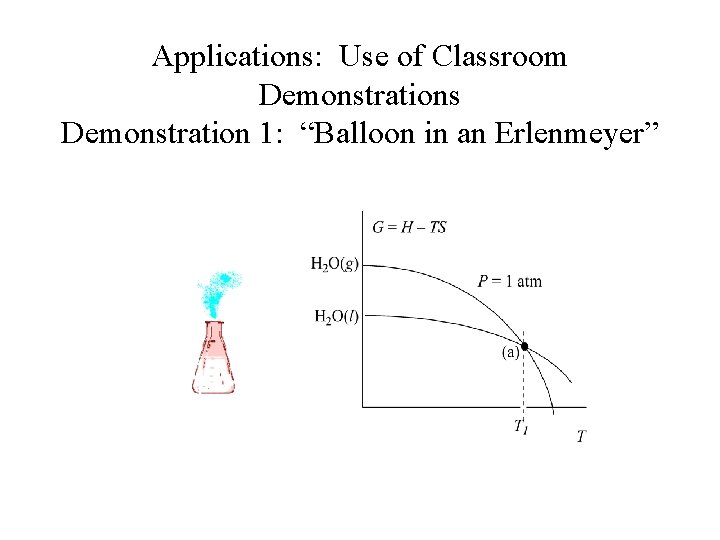
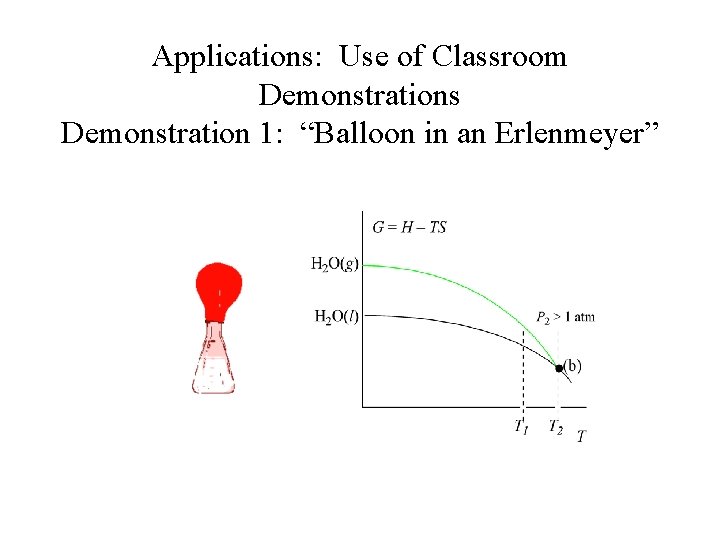
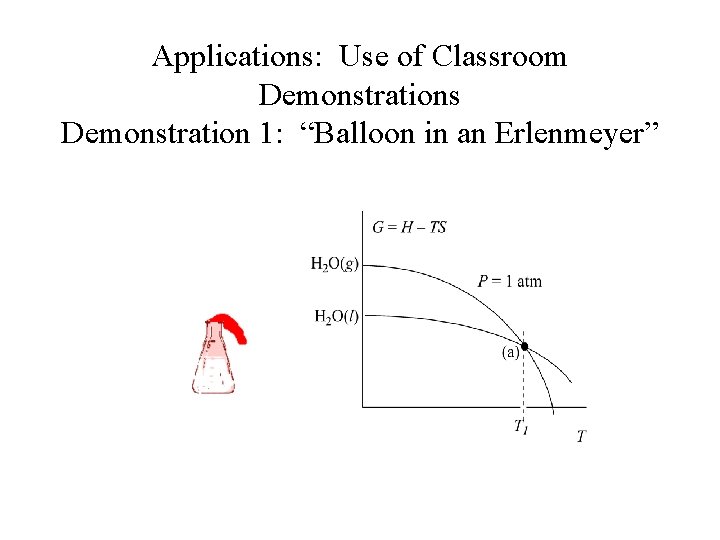
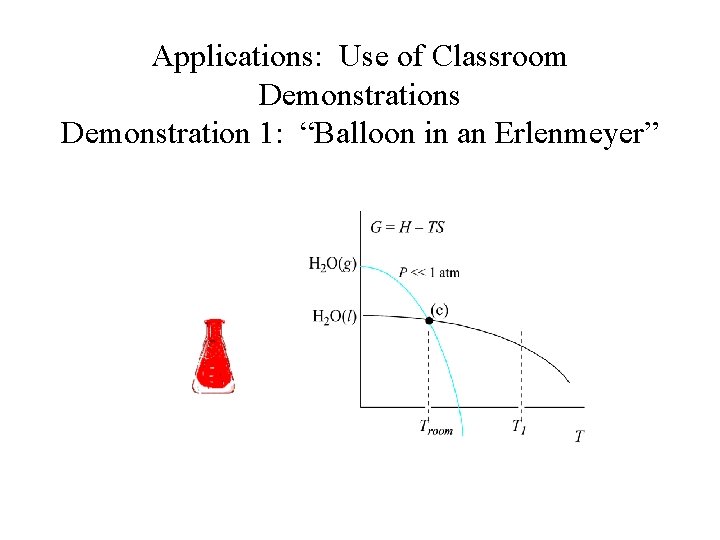
Applications: Use of Classroom Demonstrations Demonstration 1: “Balloon in an Erlenmeyer” H 2 O (l) H 2 O (g)

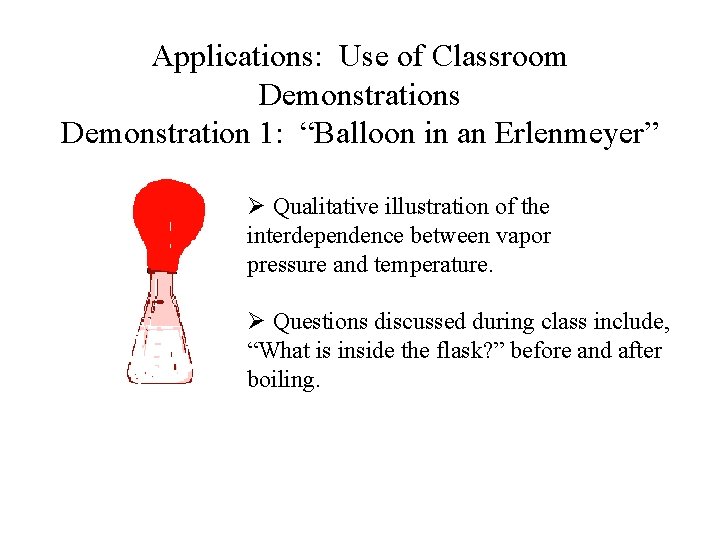
Applications: Use of Classroom Demonstrations Demonstration 1: “Balloon in an Erlenmeyer” Ø Qualitative illustration of the interdependence between vapor pressure and temperature. Ø Questions discussed during class include, “What is inside the flask? ” before and after boiling.

Applications: Use of Classroom Demonstrations Demonstration 1: “Balloon in an Erlenmeyer”

Applications: Use of Classroom Demonstrations Demonstration 1: “Balloon in an Erlenmeyer”

Applications: Use of Classroom Demonstrations Demonstration 1: “Balloon in an Erlenmeyer”

Applications: Use of Classroom Demonstrations Demonstration 1: “Balloon in an Erlenmeyer”

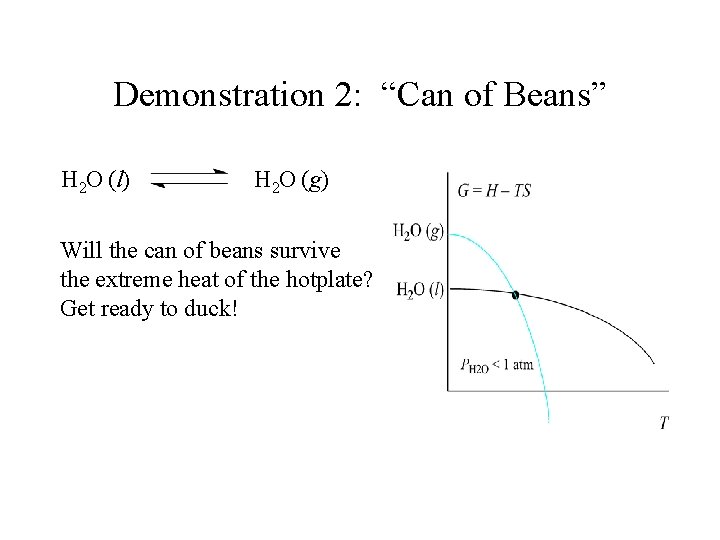
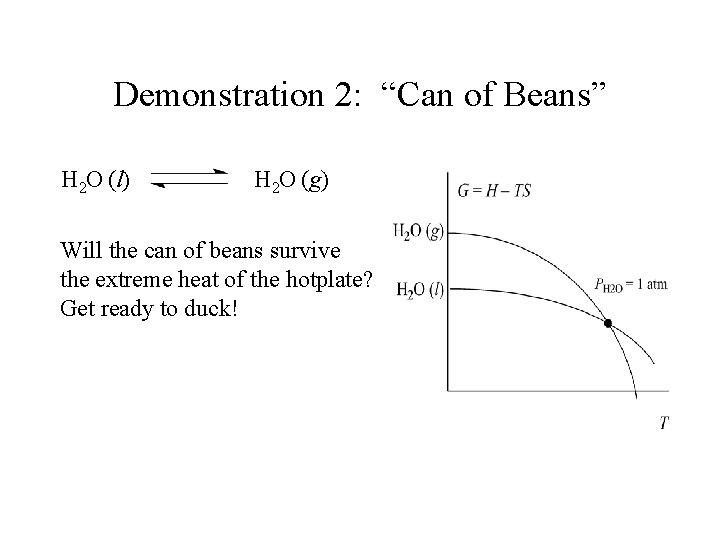
Demonstration 2: “Can of Beans” H 2 O (l) H 2 O (g) Will the can of beans survive the extreme heat of the hotplate? Get ready to duck!

Demonstration 2: “Can of Beans” H 2 O (l) H 2 O (g) Will the can of beans survive the extreme heat of the hotplate? Get ready to duck!

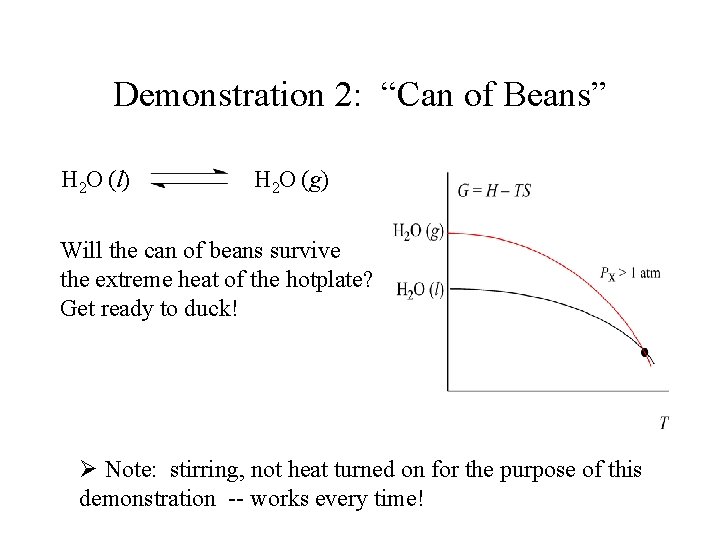
Demonstration 2: “Can of Beans” H 2 O (l) H 2 O (g) Will the can of beans survive the extreme heat of the hotplate? Get ready to duck! Ø Note: stirring, not heat turned on for the purpose of this demonstration -- works every time!

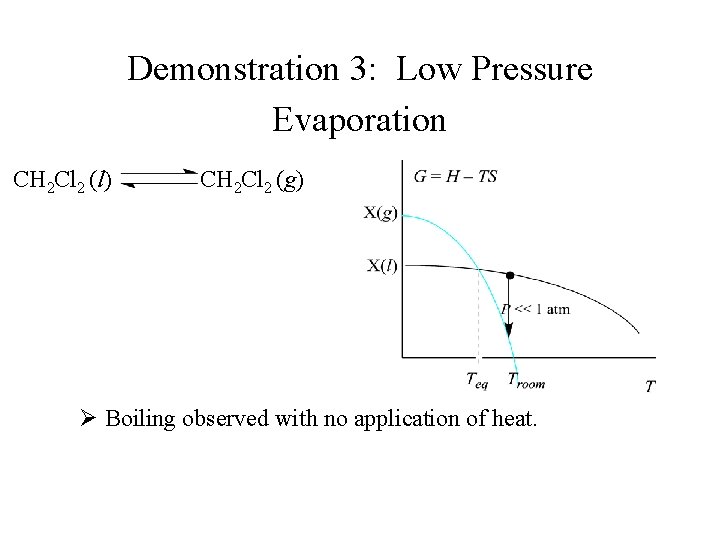
Demonstration 3: Low Pressure Evaporation CH 2 Cl 2 (l) CH 2 Cl 2 (g) Ø Boiling observed with no application of heat.

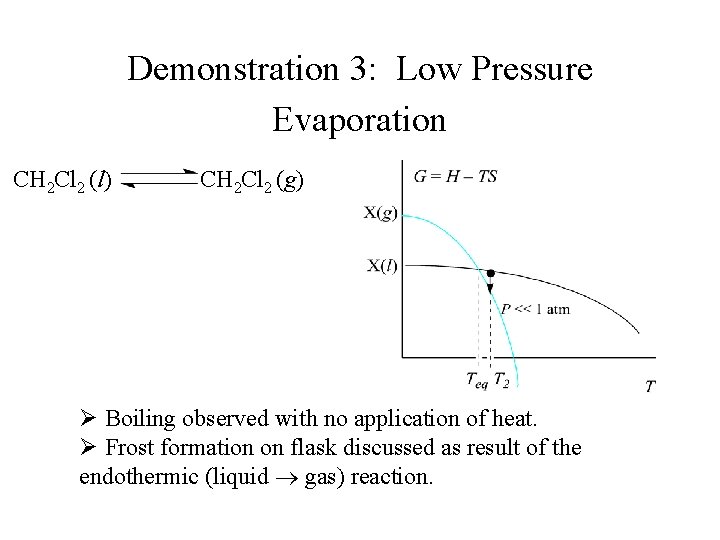
Demonstration 3: Low Pressure Evaporation CH 2 Cl 2 (l) CH 2 Cl 2 (g) Ø Boiling observed with no application of heat. Ø Frost formation on flask discussed as result of the endothermic (liquid gas) reaction.

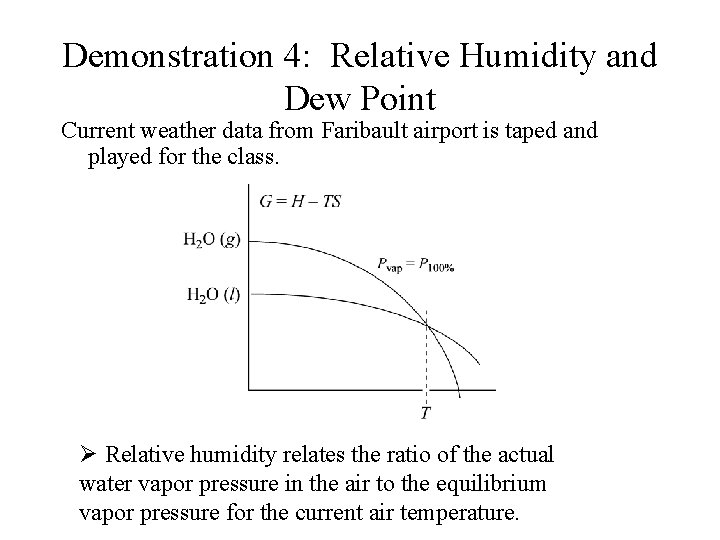
Demonstration 4: Relative Humidity and Dew Point Current weather data from Faribault airport is taped and played for the class. Ø Relative humidity relates the ratio of the actual water vapor pressure in the air to the equilibrium vapor pressure for the current air temperature.

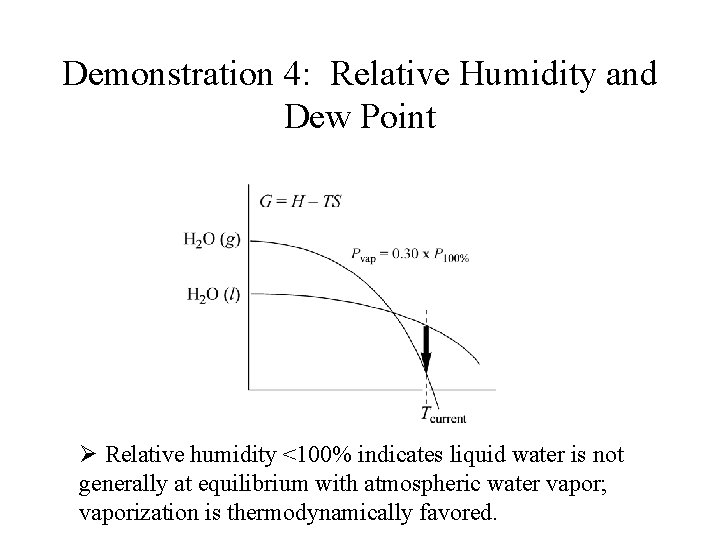
Demonstration 4: Relative Humidity and Dew Point Ø Relative humidity <100% indicates liquid water is not generally at equilibrium with atmospheric water vapor; vaporization is thermodynamically favored.

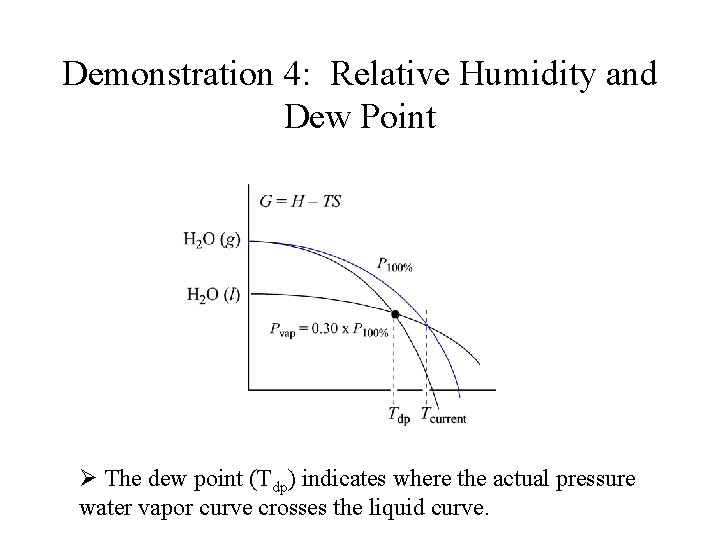
Demonstration 4: Relative Humidity and Dew Point Ø The dew point (Tdp) indicates where the actual pressure water vapor curve crosses the liquid curve.

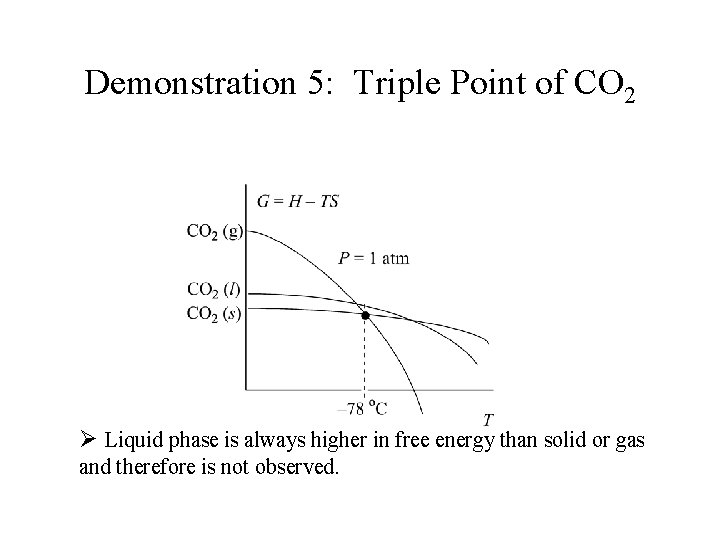
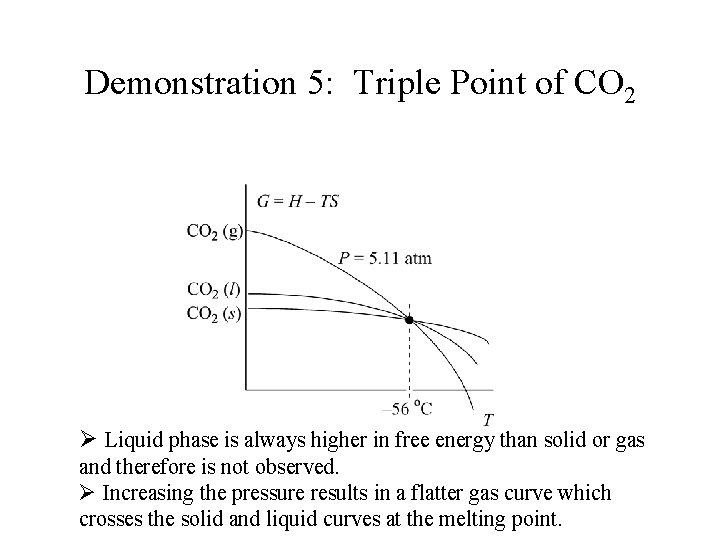
Demonstration 5: Triple Point of CO 2 Ø Liquid phase is always higher in free energy than solid or gas and therefore is not observed.

Demonstration 5: Triple Point of CO 2 Ø Liquid phase is always higher in free energy than solid or gas and therefore is not observed. Ø Increasing the pressure results in a flatter gas curve which crosses the solid and liquid curves at the melting point.

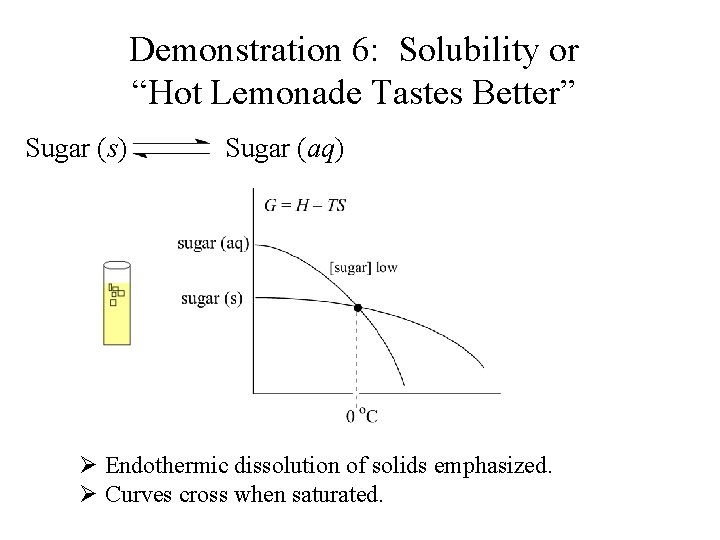
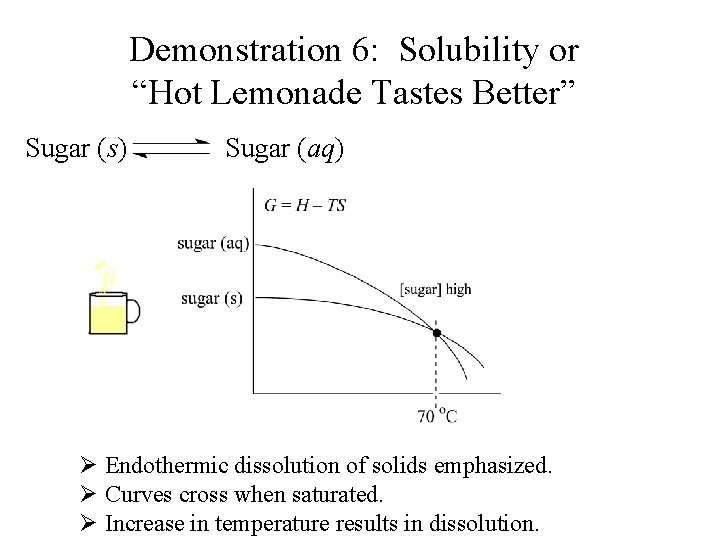
Demonstration 6: Solubility or “Hot Lemonade Tastes Better” Sugar (s) Sugar (aq) Ø Endothermic dissolution of solids emphasized.

Demonstration 6: Solubility or “Hot Lemonade Tastes Better” Sugar (s) Sugar (aq) Ø Endothermic dissolution of solids emphasized. Ø Curves cross when saturated.

Demonstration 6: Solubility or “Hot Lemonade Tastes Better” Sugar (s) Sugar (aq) Ø Endothermic dissolution of solids emphasized. Ø Curves cross when saturated. Ø Increase in temperature results in dissolution.

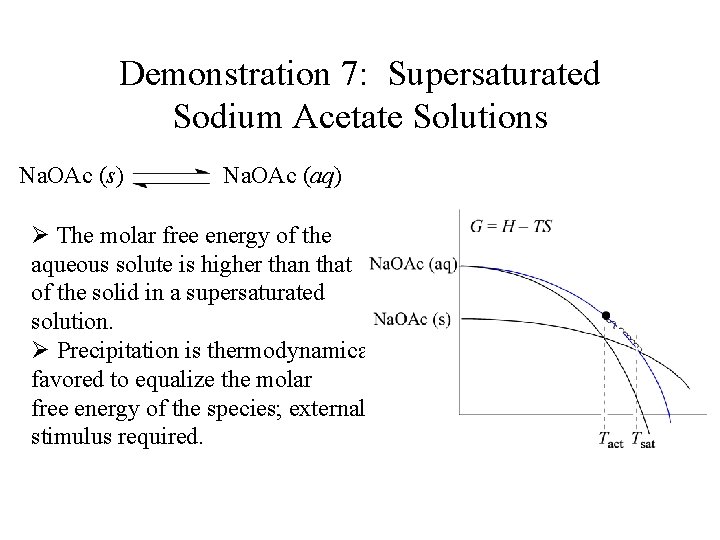
Demonstration 7: Supersaturated Sodium Acetate Solutions Na. OAc (s) Na. OAc (aq) Ø The molar free energy of the aqueous solute is higher than that of the solid in a supersaturated solution. Ø Precipitation is thermodynamically favored to equalize the molar free energy of the species; external stimulus required.

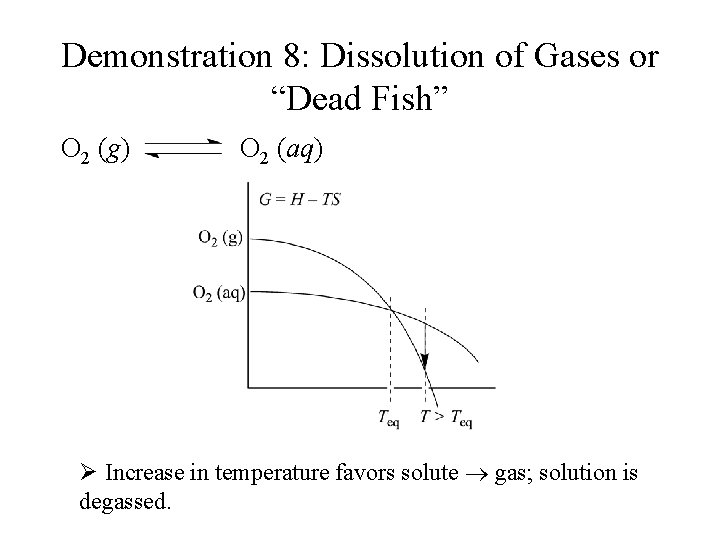
Demonstration 8: Dissolution of Gases or “Dead Fish” O 2 (g) O 2 (aq) Ø Increase in temperature favors solute gas; solution is degassed.

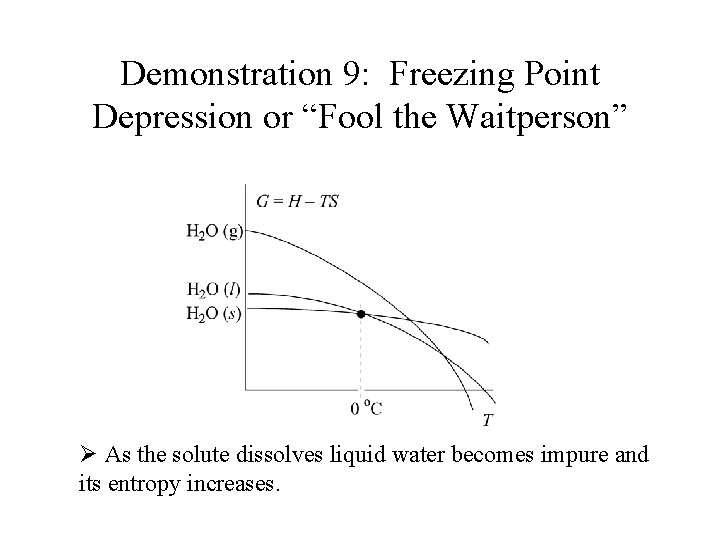
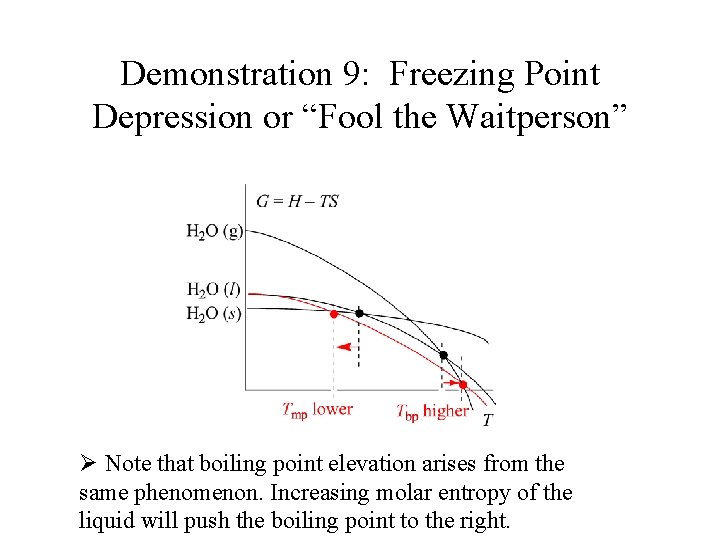
Demonstration 9: Freezing Point Depression or “Fool the Waitperson” Ø As the solute dissolves liquid water becomes impure and its entropy increases.

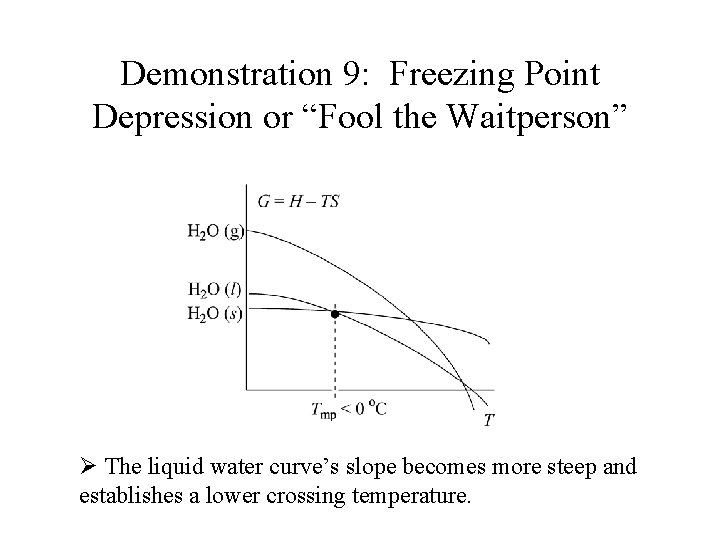
Demonstration 9: Freezing Point Depression or “Fool the Waitperson” Ø The liquid water curve’s slope becomes more steep and establishes a lower crossing temperature.

Demonstration 9: Freezing Point Depression or “Fool the Waitperson” Ø Note that boiling point elevation arises from the same phenomenon. Increasing molar entropy of the liquid will push the boiling point to the right.

Laboratory Exercise: “Chemical Magic: Free Energy” Why should chemistry professors have all the fun? Students perform a demonstration in groups of 2 -3, answer 4 -6 questions about the demonstration then move on to the next station. G-T graphs are drawn for each laboratory exercise.

Laboratory Exercise: “Chemical Magic: Free Energy” Laboratory demonstrations include: “Inside-Out Balloon” “Fog Chamber” “Cold Boiling” “Liquid Air” “Cool Glue” “Hot Ice” “Crystals from not-so-thin-air” “Instant Snowflakes” “Instant Hot; Instant Cold” “Magic Rope” Upon completing the third demonstration (45 minutes) the students prepare to present a magic show for their classmates.

Laboratory Exercise: “Chemical Magic: Free Energy”

Laboratory Exercise: “Chemical Magic: Free Energy” Ø “Chemical Magic is fun!”

Conclusion Ø Free energy vs. temperature graphs can be utilized to discuss a variety of common classroom demonstrations. Ø “Chemical Magic” laboratory exercise can effectively supplement the discussion initiated during “Demo-Days. ”
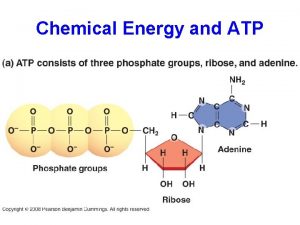
 Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Q chemistry
Q chemistry How to calculate gibbs free energy
How to calculate gibbs free energy Helmholtz free energy
Helmholtz free energy Ornamental horticulture demonstrations ideas
Ornamental horticulture demonstrations ideas Political demonstrations on american campuses have abated
Political demonstrations on american campuses have abated Phân độ lown
Phân độ lown Block xoang nhĩ là gì
Block xoang nhĩ là gì Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Quale fu il rapporto tra i tiranni è il demos
Quale fu il rapporto tra i tiranni è il demos Bigelow y lombard
Bigelow y lombard Kratein definition
Kratein definition Atmosfer berasal dari kata
Atmosfer berasal dari kata Demos kratos greek
Demos kratos greek Biomotion lab demos
Biomotion lab demos The mother of all demos
The mother of all demos Demos.com/calculator
Demos.com/calculator Flash demo
Flash demo Epi demos logos
Epi demos logos Demos kratein
Demos kratein 이중결합요소
이중결합요소 Demos
Demos Epi demos logos
Epi demos logos As a roller coaster goes downhill
As a roller coaster goes downhill ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Chemical potential energy examples pictures
Chemical potential energy examples pictures Photosynthesis transforms light energy into chemical energy
Photosynthesis transforms light energy into chemical energy Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Typiska novell drag
Typiska novell drag