Demos Electrostatic Motor Large Capcitor and Quarters small

















- Slides: 35

Demos ► Electrostatic Motor ► Large Capcitor and Quarters (small scale lightening rod) ► Light Bulb Board ► Nichrome Wire ► Hot-dog cooker ► 12 V battery and bulb vs. 120 V bulb ► Vinegar Battery

Electrical Energy a) b) c) d) Easy to Generate Easy to Transmit Can be 100% renewable and nonpolluting. Easy to change into different forms of energy. 1 2 3 4 5 Heating Elements Motors (Rotational Kinetic Energy) Lighting and other E&M Waves Sound Waves (e. g. speakers) Actuators (electrical signals used to actuate something mechanically)

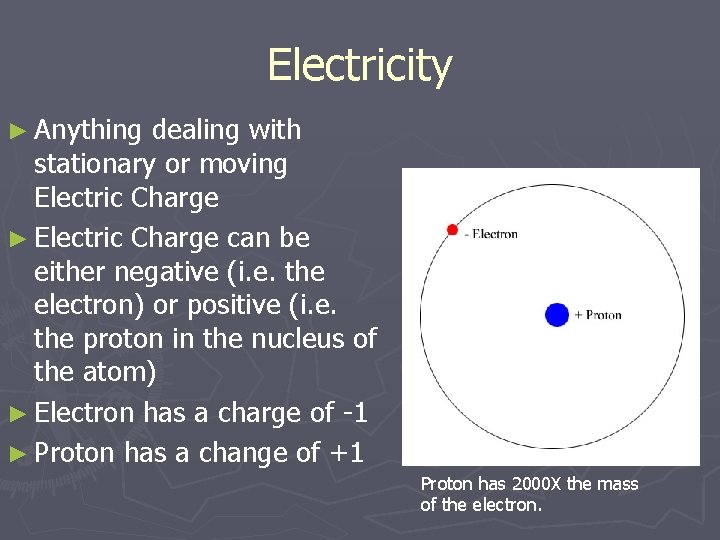
Electricity ► Anything dealing with stationary or moving Electric Charge ► Electric Charge can be either negative (i. e. the electron) or positive (i. e. the proton in the nucleus of the atom) ► Electron has a charge of -1 ► Proton has a change of +1 Proton has 2000 X the mass of the electron.

Electricity… prior to 18 th-century people experienced static electricity and naturally occurring electricity (lightening) but didn’t understand it. In 1663 a device similar to the Van de Graph generator seen in lab was created.

Ben Franklin’s Kite Experiment Ben Franklin thought the sparks from static electricity looked a lot like lightning. A metal key was attached to the kite’s string. The key was attached to a Leyden Jar via a small wire. It is unlikely Franklin ever did the legendary experiment because other people who tried the experiment had fatal results. The installation of Lightening Rods to protect tall buildings came from Franklin.

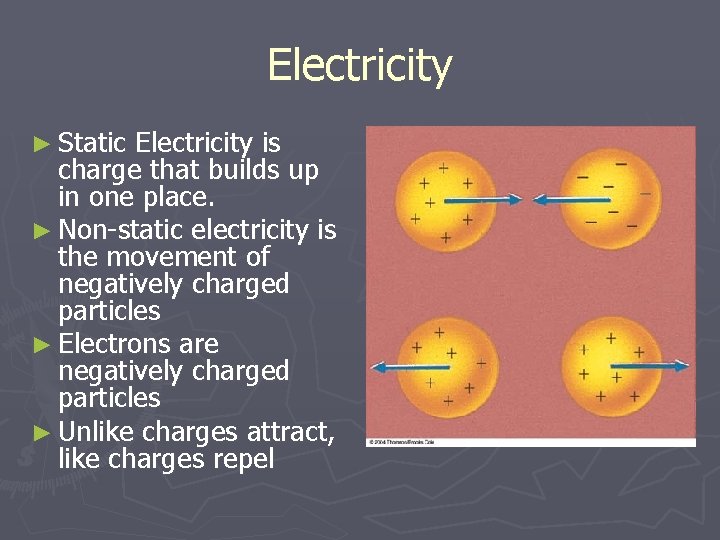
Electricity ► Static Electricity is charge that builds up in one place. ► Non-static electricity is the movement of negatively charged particles ► Electrons are negatively charged particles ► Unlike charges attract, like charges repel

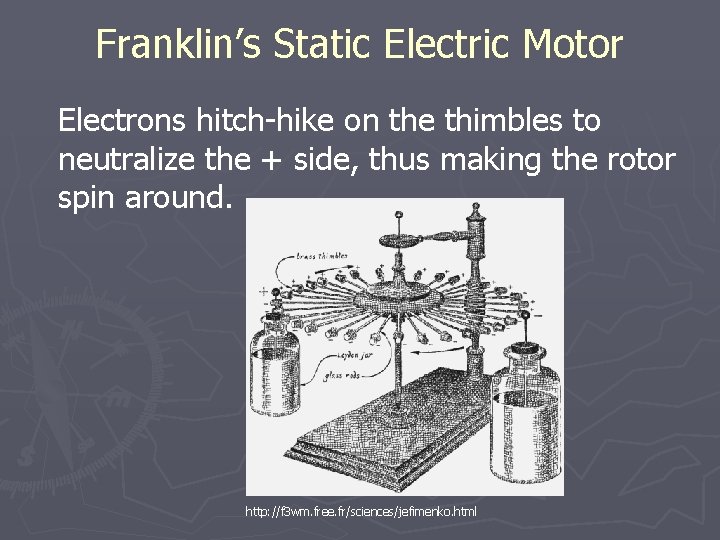
Franklin’s Static Electric Motor Electrons hitch-hike on the thimbles to neutralize the + side, thus making the rotor spin around. http: //f 3 wm. free. fr/sciences/jefimenko. html

What is a electrical conductor? ►A material that allows electrons to flow freely. Most metals are good electrical conductors. Outer electrons are loosely bound to the metal atoms, therefore they can roam freely. “Cloud of Electrons” ► A material that strongly resists the flow of electrons is an insulator. Most non-metals are good insulators. ► A semiconductor can be made into a conductor or an insulator. It’s electrical properties can be precisely controlled with added impurities.

Unit of Charge ► Coulomb ► 1 C = 6 Billion, Billion electrons ► 6. 241506 x 1018 electrons

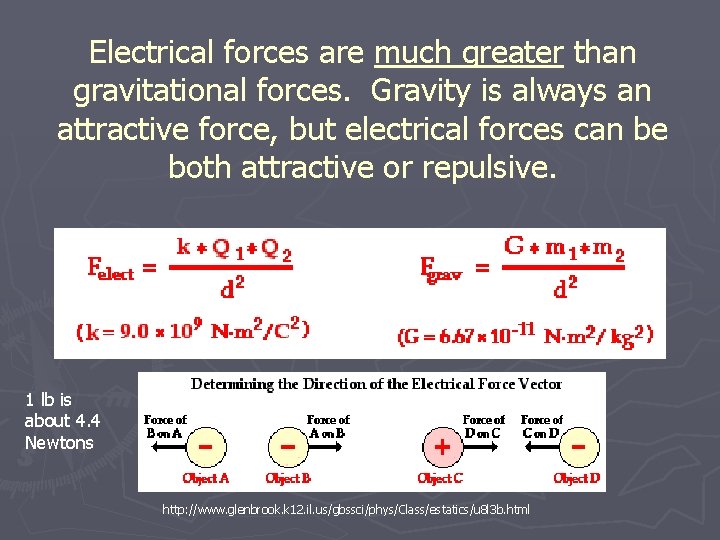
Electrical forces are much greater than gravitational forces. Gravity is always an attractive force, but electrical forces can be both attractive or repulsive. 1 lb is about 4. 4 Newtons http: //www. glenbrook. k 12. il. us/gbssci/phys/Class/estatics/u 8 l 3 b. html

Electrostatic forces can be stronger than the force of gravity. www. wfu. edu/physics

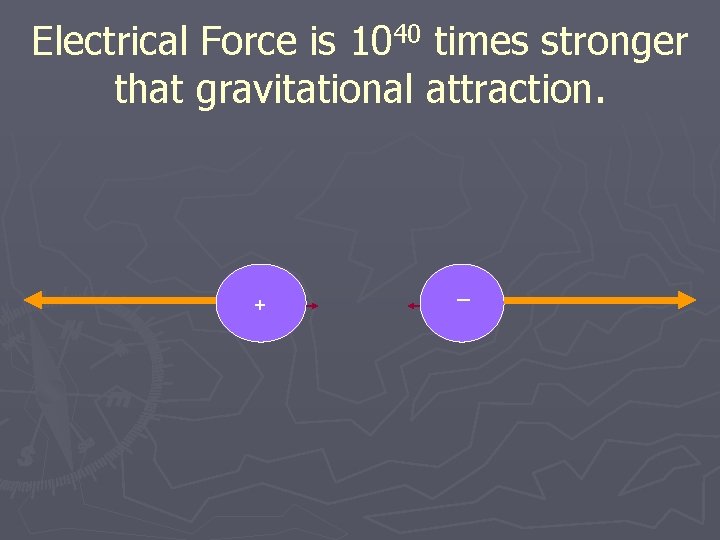
Electrical Force is 1040 times stronger that gravitational attraction. + _

Examples of Electrical Forces ► Chemical Bonding ► Forces that keep materials together ► Burning Gas ► Energy in a Spring ► Friction

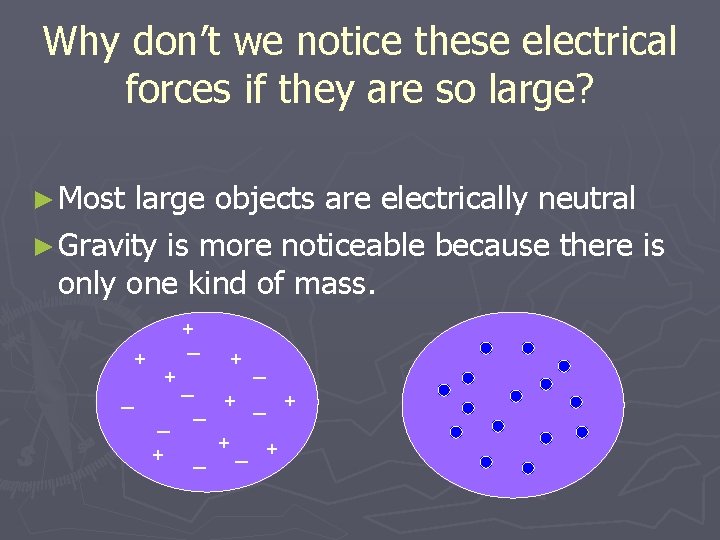
Why don’t we notice these electrical forces if they are so large? ► Most large objects are electrically neutral ► Gravity is more noticeable because there is only one kind of mass. + + _ _ _ + +

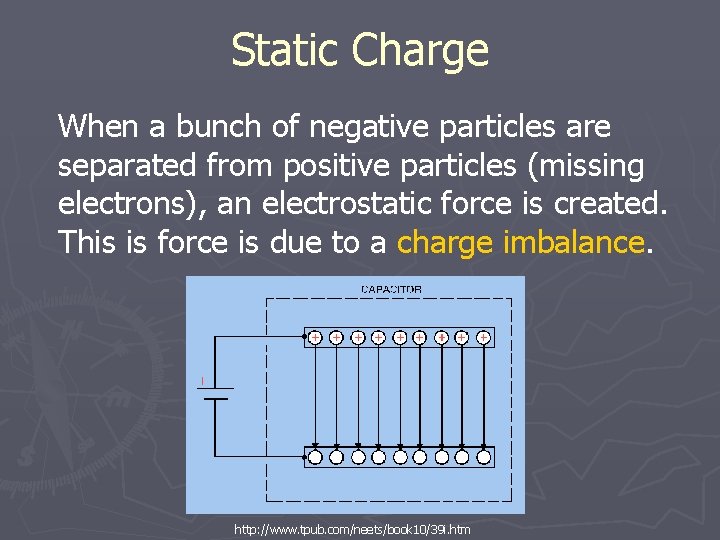
Static Charge When a bunch of negative particles are separated from positive particles (missing electrons), an electrostatic force is created. This is force is due to a charge imbalance. http: //www. tpub. com/neets/book 10/39 i. htm

Van De Graph Generator produces static charge

Photocopier Process http: //www. howstuffworks. com/photocopier 1. htm Photocopier Drums are light sensitive. This photoconductive material becomes conductive when in light, but not conductive in the dark. Charge can leave the drum where light hits the drum. Charge remains in the areas where light did not shine.

Xerographic Copies use electrostatic charge to produce & transfer images ►A document image is projected onto a rotating photoconductor drum. An image identical to the original black and white is produced with charged particles on the drum. ► Oppositely charged toner adheres to the charged portion of the photoconductor. ► A charged piece of paper makes contact with the photoconductor and the toner image is transferred to the paper. ► The paper is then heated and the toner is fused onto the paper. http: //www. sciam. com/article. cfm? id=how-does-a-photocopier-wo

Chester Carlson Physicist, Engineer, & Patent Lawyer ► Develops the electrophotography process in 1938 ► Acquires patents for the process ► Seeks investors from IBM, RCA, General Electric, and the US Army ► Finally in 1944 convinces Haloid Co. that idea has commercial viability. ► Process is coined Xero. X, and Haloid Co. changes its name to Xerox and begins selling copiers in 1950 ► Plain Paper copies come out in 1959; sales surge along with Xerox’s revenue

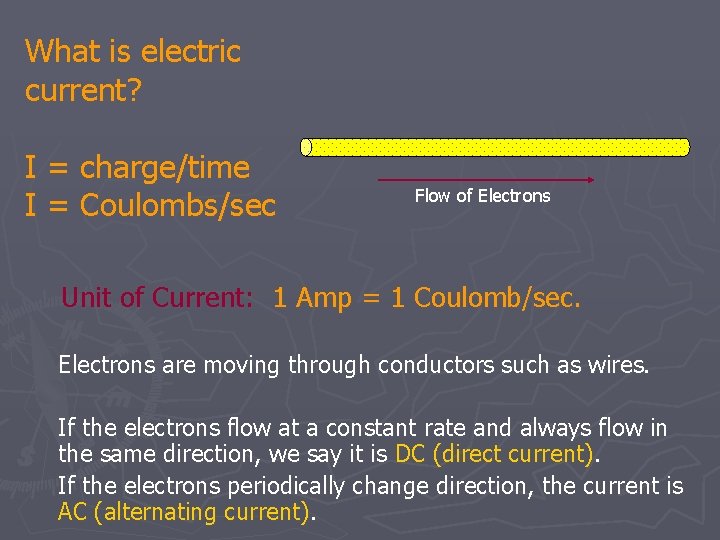
What is electric current? I = charge/time I = Coulombs/sec Flow of Electrons Unit of Current: 1 Amp = 1 Coulomb/sec. Electrons are moving through conductors such as wires. If the electrons flow at a constant rate and always flow in the same direction, we say it is DC (direct current). If the electrons periodically change direction, the current is AC (alternating current).

When do we get current? ► Cloud of Electrons in conductors respond to an Electric Field. ► An Electric field is similar to a gravitational field. ► Mass responds to gravity ► Electrons respond to an Electric Field ► The Electric Field Potential is measured in Volts

The amount of Electric Field needed to move electrons in a wire depends on… ► How much current we want ► The resistance of the material that the electrons have to travel through

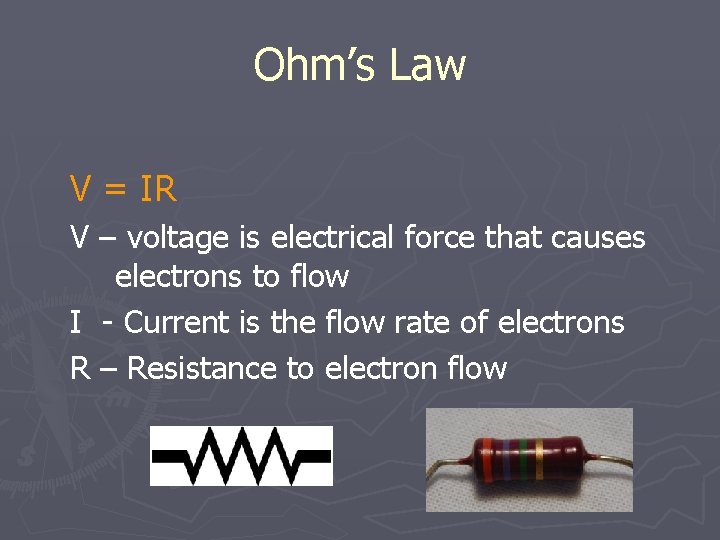
Ohm’s Law V = IR V – voltage is electrical force that causes electrons to flow I - Current is the flow rate of electrons R – Resistance to electron flow

Voltage Potential 1 jolt = 1 joule/1 coulomb

Current in the Nichrome wire Electrical Energy is converted into light and heat by passing current through a resistive wire.

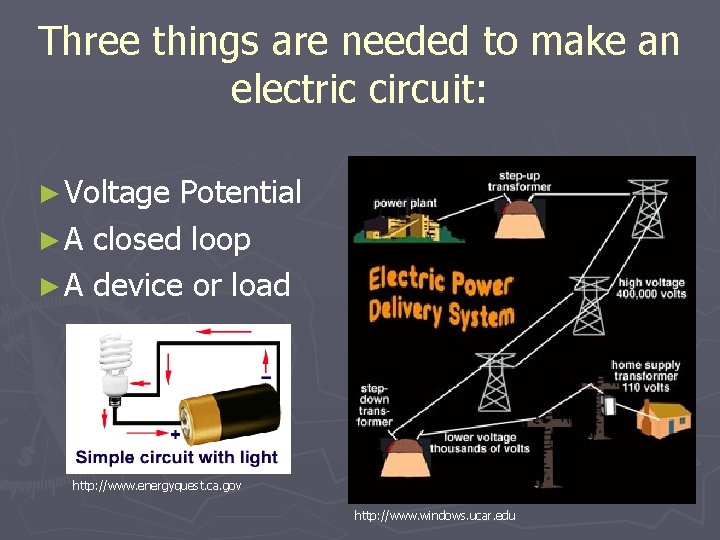
Three things are needed to make an electric circuit: ► Voltage Potential ► A closed loop ► A device or load http: //www. energyquest. ca. gov http: //www. windows. ucar. edu

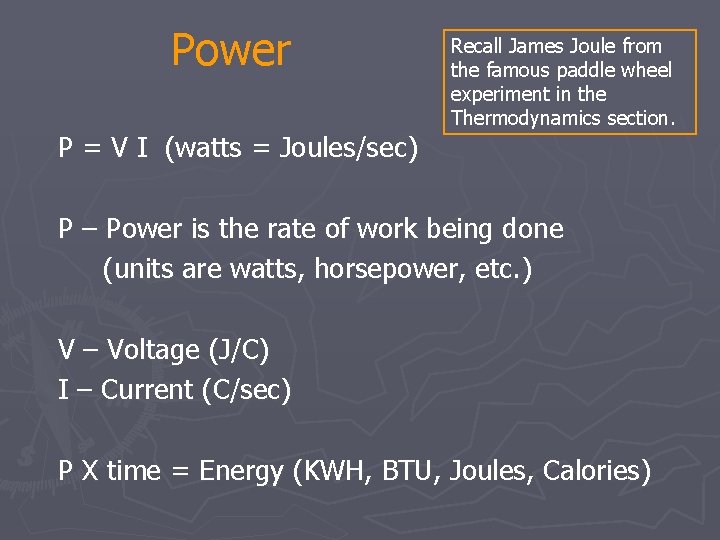
Power P = V I (watts = Joules/sec) Recall James Joule from the famous paddle wheel experiment in the Thermodynamics section. P – Power is the rate of work being done (units are watts, horsepower, etc. ) V – Voltage (J/C) I – Current (C/sec) P X time = Energy (KWH, BTU, Joules, Calories)

To perform work, a continuous flow of charge is required Some kind of device or system is needed to produce a continuous flow of charge.

Luigi Galvani’s conducts experiments with “animal electricity” using frogs’ legs Applying static electricity to dissected frogs’ legs caused the legs to twitch. (c. 1780) Luigi Galvani http: //itp. nyu. edu

Galvani hanging frog legs on brass hooks. www. hgs. k 12. va. us Two metals causing legs To twitch. www. karisteeves. net Galvani later laid frog legs out on brass hooks that were hooked onto an iron lattice during a thunderstorm. The frog legs continued to twitch after the storm.

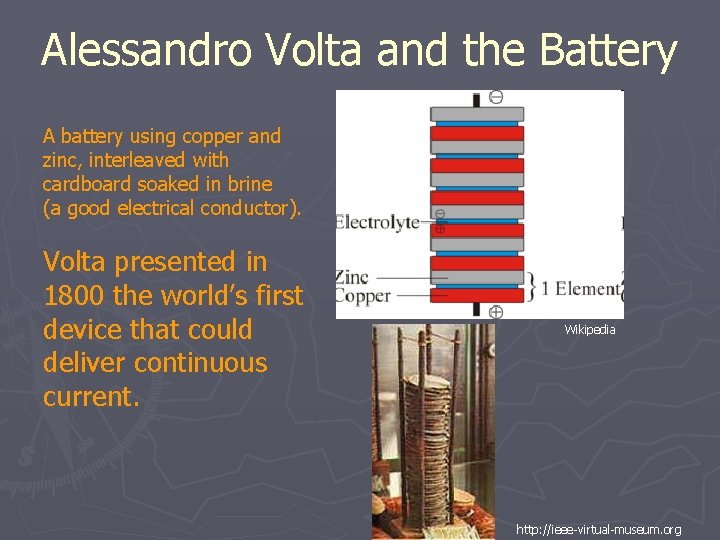
Alessandro Volta and the Battery A battery using copper and zinc, interleaved with cardboard soaked in brine (a good electrical conductor). Volta presented in 1800 the world’s first device that could deliver continuous current. Wikipedia http: //ieee-virtual-museum. org

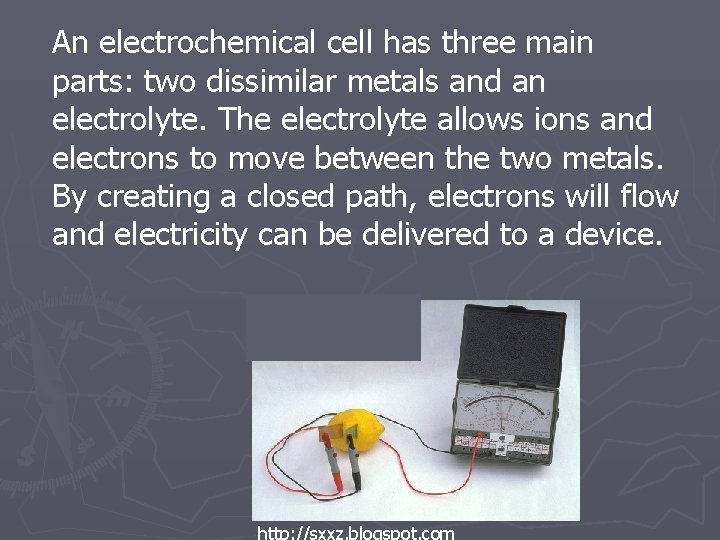
An electrochemical cell has three main parts: two dissimilar metals and an electrolyte. The electrolyte allows ions and electrons to move between the two metals. By creating a closed path, electrons will flow and electricity can be delivered to a device.

The Carbon and Zinc dry-cell battery First demonstrated in 1866. This is a chemical reaction where electrons are exchanged between two metals, causing continuous current through a load. Wikipedia

Lead Acid Battery Reactions Pb + SO 4²¯ → Pb. SO 4 + 2 e- + H+ The reduction potential of this reaction is +0. 356 Volts. Pb. O 2 + SO 4²¯ + 4 H+ + 2 e- → Pb. SO 4 + H 2 O The potential of this reaction is 1. 685 volts. Total voltage created is about 2 V. Lead Acid batteries provide high current, and are rechargeable, making them effective for starting cars. http: //sxxz. blogspot. com/2005/03/how-do-batteries-work. html

Text Sources ► The Sciences- 5 e, Trefil and Hazen ► A History of Great Inventions, James Dyson ► Internet