Dementia MUDr Tom Kaprek Dep of Psychiatry Masaryk


























- Slides: 41

Dementia MUDr. Tomáš Kašpárek Dep. of Psychiatry Masaryk University, Brno

Contents Definition and clinical manifestation Classification Alzheimer's disease and its treatment Other causes of dementia

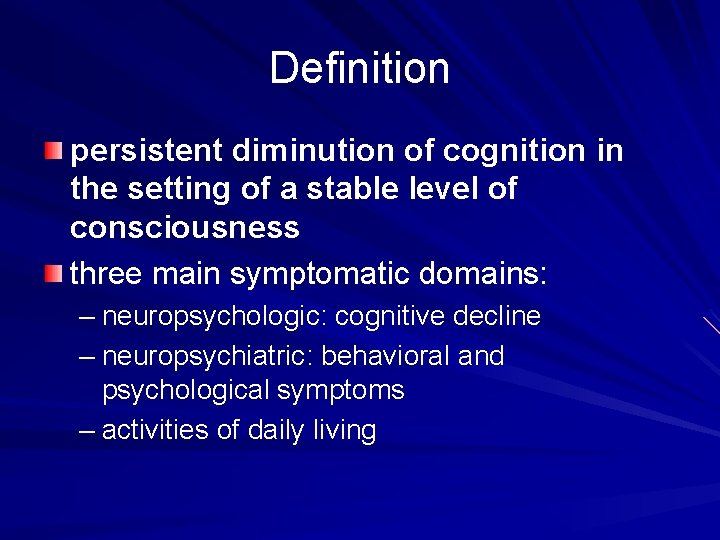
Definition persistent diminution of cognition in the setting of a stable level of consciousness three main symptomatic domains: – neuropsychologic: cognitive decline – neuropsychiatric: behavioral and psychological symptoms – activities of daily living

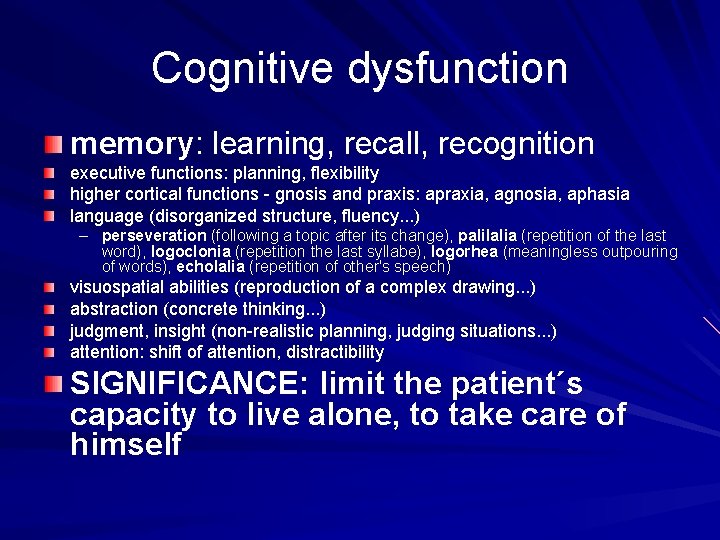
Cognitive dysfunction memory: learning, recall, recognition executive functions: planning, flexibility higher cortical functions - gnosis and praxis: apraxia, agnosia, aphasia language (disorganized structure, fluency. . . ) – perseveration (following a topic after its change), palilalia (repetition of the last word), logoclonia (repetition the last syllabe), logorhea (meaningless outpouring of words), echolalia (repetition of other's speech) visuospatial abilities (reproduction of a complex drawing. . . ) abstraction (concrete thinking. . . ) judgment, insight (non-realistic planning, judging situations. . . ) attention: shift of attention, distractibility SIGNIFICANCE: limit the patient´s capacity to live alone, to take care of himself

Behavioral and psychological symptoms of dementia emotional disturbances: depression, anxiety, irritability paranoid ideation hallucinations agression wandering circadian rhythm disturbance (wake-sleep) SIGNIFICANCE: limit the possibility of family care and patient´s well being

Disruption of activities of daily livnig basic activities – – dressing eating washing excretory/toilet activities instrumental activities – household tasks SIGNIFICANCE: need of care

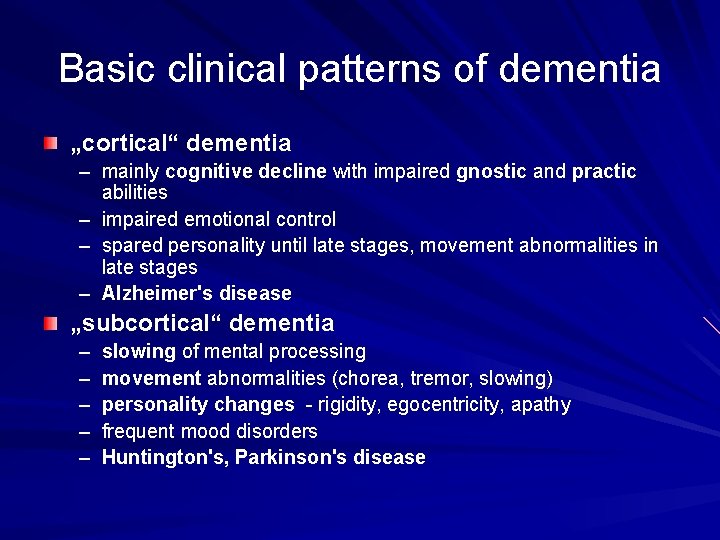
Basic clinical patterns of dementia „cortical“ dementia – mainly cognitive decline with impaired gnostic and practic abilities – impaired emotional control – spared personality until late stages, movement abnormalities in late stages – Alzheimer's disease „subcortical“ dementia – – – slowing of mental processing movement abnormalities (chorea, tremor, slowing) personality changes - rigidity, egocentricity, apathy frequent mood disorders Huntington's, Parkinson's disease

Classification Dementia of the Alzheimer´s type Vascular dementia Dementia due to other general medical conditions Substance-induced persisting dementia Dementia due to multiple etiologies Dementia not otherwise specified

Causes of dementia Degenerative dementias: Alzheimer's, Pick's, Parkinson's, Wilson's disease Cardiac/vascular: single/multiple infarction, lacunar infarction, Binswanger´s disease Congenital/hereditary: Huntington's disease Infection: Syphilis, Creutzfeld-Jacob disease, AIDS Physiological: epilepsy, normal pressure hydrocephalus Trauma: posttraumatic dementia, hematomas Metabolic: vitamin deficiencies (B 12, folate), chronic metabolic disturbances, chronic anoxic states, chronic endocrinopathies Tumors Demyelinating: multiple sclerosis Drugs and toxins: alcohol, heavy metal, carbon monooxid

Alzheimer's disease

Characteristics „cortical“ dementia 50 -60% of all dementias familial vs. sporadic, early-onset (<65) vs. lateonset prevalence: 6 -9% in general population, doubles every 10 years, ½ of aged 85 years risk factors = APOE 4, high age protective factors = higher education, larger head circumference, smoking.

Diagnosis definite diagnosis = neuropathology – generalized cortical atrophy – neurofibrillary tangles (in neurons) – neuritic senile plaques (amyloid) clinical diagnosis = exclusion of other etiologies – no pathognomic laboratory or imaging findings (low specificity of laboratory tests - tau protein, Aβ? )

DSM IV Diagnostic criteria A: development of multiple cognitive deficits – memory impairment – aphasia, apraxia, agnosia, impaired executive functions B: significant impairment in social or occupational functioning C: gradual onset with continuing cognitive decline D: cognitive deficits are not due – – – other CNS condition systemic condition substance induced conditions E: deficits do not occure exclusively during a delirium

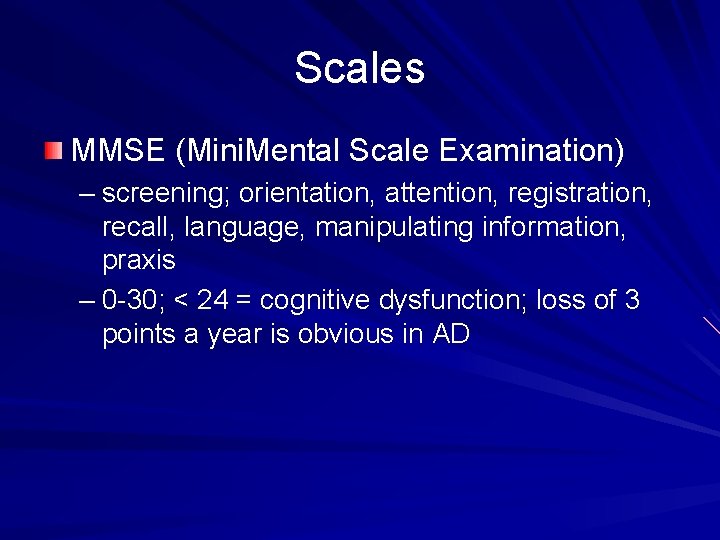
Scales MMSE (Mini. Mental Scale Examination) – screening; orientation, attention, registration, recall, language, manipulating information, praxis – 0 -30; < 24 = cognitive dysfunction; loss of 3 points a year is obvious in AD

Pathogenesis I: Senile plaques insoluble deposits of amyloid β-peptide (Aβ) – fragment of the amyloid precursor protein (APP, membrane protein – function? ) 2 pathways of metabolism/cleavage: – α secretase = nonamyloidogenic; enhanced via PKC, thus via receptors activating PKC (m 1 ACR) – β and γ secretases = Aβ – both pathways active even in the health – disease = dysregulation? – 2 types of Aβ: Aβ 42, longer, initial phase, higher aggregability; Aβ 40 neurotoxicity – direct x indirect – inflamation, production of free radicals

Pathogenesis II: neurofibrillary tangles (NFTs) amount correlates with the severity of AD (x plaques) first in transentorhinal region spreading to hippocampus and other cortical regions (x plaques) presence in neuron signals its dysfunction/death consist of aggregated tau protein – normally in axons of healthy neurons – stabilization of microtubules (essential for fast axonal transport) – regulated by phosphorylation – disturbed in AD (phosphatase A 2, glycogen-synthase kinase-3? ) = loss of its function = dysfunction of microtubules preceedes aggregation into NFTs – mutations of tau itself do not lead to amyloid accumulation, but lead to tau aggregation - fronto-temporal dementia, tauopathies

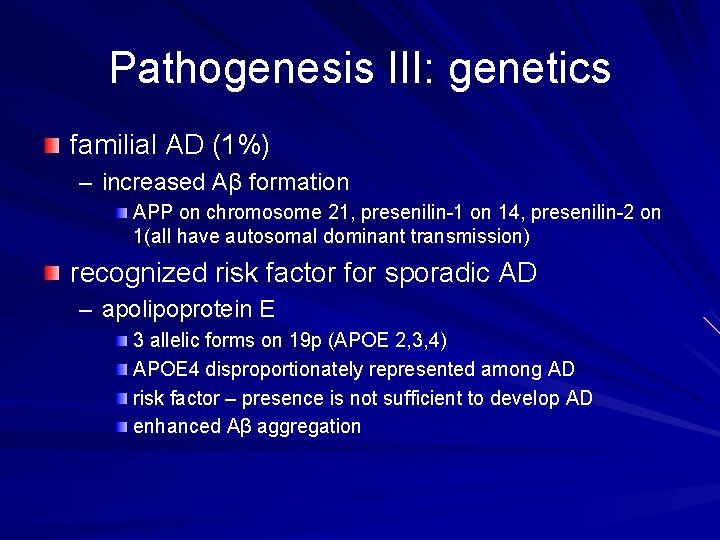
Pathogenesis III: genetics familial AD (1%) – increased Aβ formation APP on chromosome 21, presenilin-1 on 14, presenilin-2 on 1(all have autosomal dominant transmission) recognized risk factor for sporadic AD – apolipoprotein E 3 allelic forms on 19 p (APOE 2, 3, 4) APOE 4 disproportionately represented among AD risk factor – presence is not sufficient to develop AD enhanced Aβ aggregation

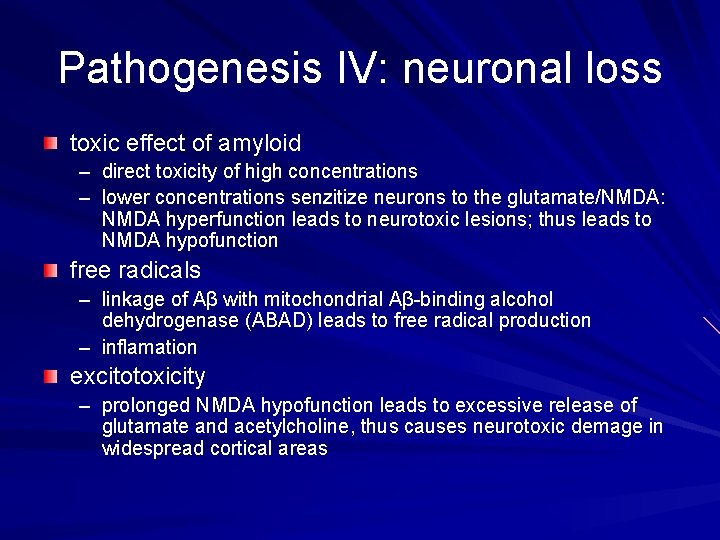
Pathogenesis IV: neuronal loss toxic effect of amyloid – direct toxicity of high concentrations – lower concentrations senzitize neurons to the glutamate/NMDA: NMDA hyperfunction leads to neurotoxic lesions; thus leads to NMDA hypofunction free radicals – linkage of Aβ with mitochondrial Aβ-binding alcohol dehydrogenase (ABAD) leads to free radical production – inflamation excitotoxicity – prolonged NMDA hypofunction leads to excessive release of glutamate and acetylcholine, thus causes neurotoxic demage in widespread cortical areas

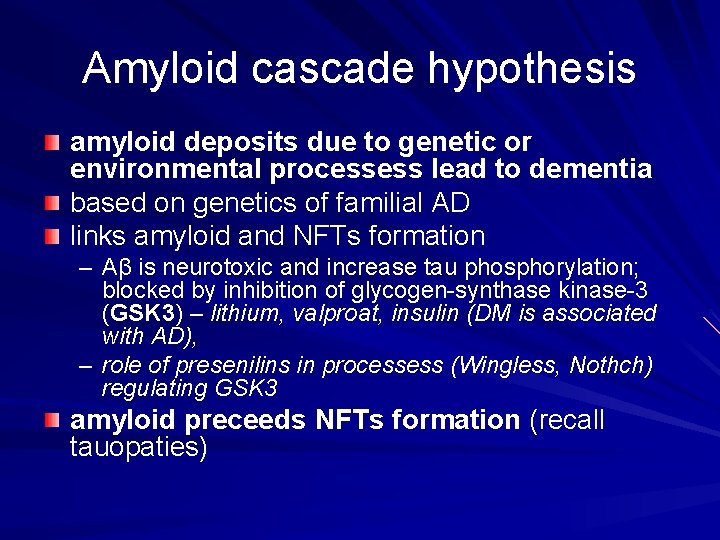
Amyloid cascade hypothesis amyloid deposits due to genetic or environmental processess lead to dementia based on genetics of familial AD links amyloid and NFTs formation – Aβ is neurotoxic and increase tau phosphorylation; blocked by inhibition of glycogen-synthase kinase-3 (GSK 3) – lithium, valproat, insulin (DM is associated with AD), – role of presenilins in processess (Wingless, Nothch) regulating GSK 3 amyloid preceeds NFTs formation (recall tauopaties)

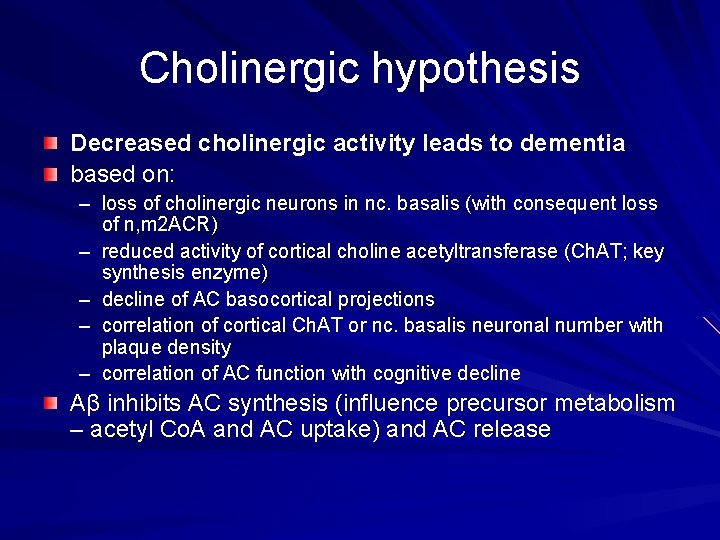
Cholinergic hypothesis Decreased cholinergic activity leads to dementia based on: – loss of cholinergic neurons in nc. basalis (with consequent loss of n, m 2 ACR) – reduced activity of cortical choline acetyltransferase (Ch. AT; key synthesis enzyme) – decline of AC basocortical projections – correlation of cortical Ch. AT or nc. basalis neuronal number with plaque density – correlation of AC function with cognitive decline Aβ inhibits AC synthesis (influence precursor metabolism – acetyl Co. A and AC uptake) and AC release

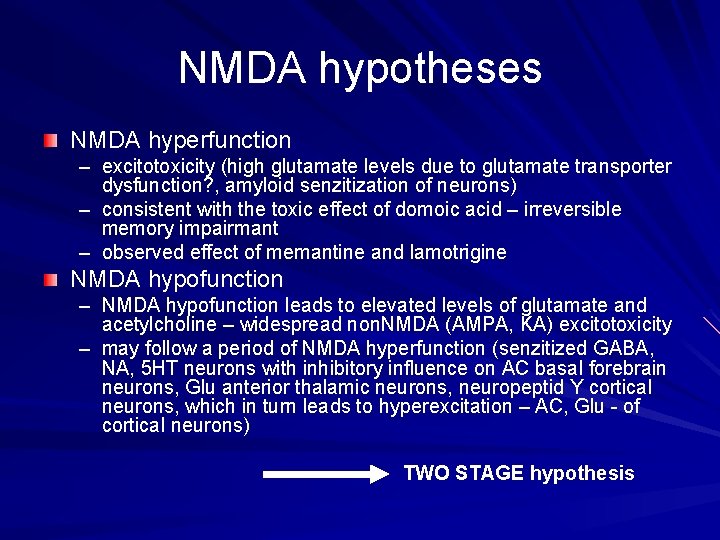
NMDA hypotheses NMDA hyperfunction – excitotoxicity (high glutamate levels due to glutamate transporter dysfunction? , amyloid senzitization of neurons) – consistent with the toxic effect of domoic acid – irreversible memory impairmant – observed effect of memantine and lamotrigine NMDA hypofunction – NMDA hypofunction leads to elevated levels of glutamate and acetylcholine – widespread non. NMDA (AMPA, KA) excitotoxicity – may follow a period of NMDA hyperfunction (senzitized GABA, NA, 5 HT neurons with inhibitory influence on AC basal forebrain neurons, Glu anterior thalamic neurons, neuropeptid Y cortical neurons, which in turn leads to hyperexcitation – AC, Glu - of cortical neurons) TWO STAGE hypothesis

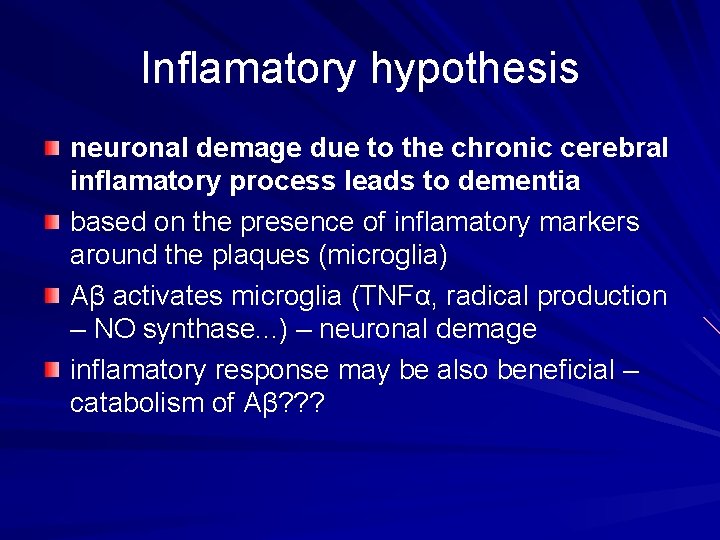
Inflamatory hypothesis neuronal demage due to the chronic cerebral inflamatory process leads to dementia based on the presence of inflamatory markers around the plaques (microglia) Aβ activates microglia (TNFα, radical production – NO synthase. . . ) – neuronal demage inflamatory response may be also beneficial – catabolism of Aβ? ? ?

Treatment I Psychosocial treatment – help patients remain in their homes – environmental manipulation reminders, clocks, calendars, regular activities – prevention of comorbidity adequate hydration, nutrition, excercise, cleanliness – family support exhaustion, depression, anxiety, insomnia

Treatment II - Pharmacoterapy Cognitive disturbance – Cholinesterase inhibitors (Ch. EIs) – Memantine - NMDA antagonists behavioral disturbance – antipsychotics, antidepressants; beware adverese effects

Cholinesterase inhibitors I cholinesterases: acetylcholinesterase (ACh. E), butyrylcholinexterase (BChe)– hydrolysis of acetylcholin, thus decrease its amount in synapses molecular forms of AChe – G 4 (tetramer) – presynaptic membrane – both hydrolysis and feedback inhibition; decrease in AD and aging – G 1 (monomer) – postsynaptic membrane; no significant decrease

Cholinesterase inhibitors II donepezil – long-acting, selective, reversible ACh. EI – metabolized by the liver microsome syst. rivastigmin – pseudo-irreversible, both ACh. EI (G 1) and BCh. EI – no liver microsome metabolism galantamin – reversibilie, competitive (increases AC only in areas with low AC concentration – lower central cholinergic side effects than noncompetitive inhibitors) ACh. EI + allosteric modulation n. ACR of

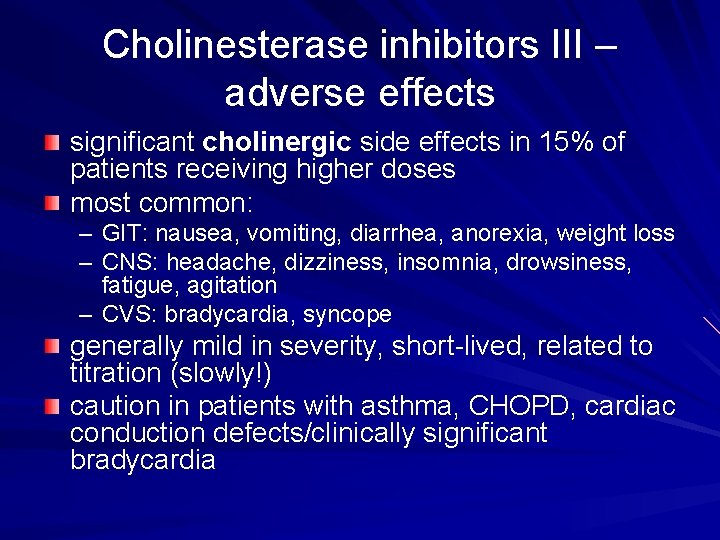
Cholinesterase inhibitors III – adverse effects significant cholinergic side effects in 15% of patients receiving higher doses most common: – GIT: nausea, vomiting, diarrhea, anorexia, weight loss – CNS: headache, dizziness, insomnia, drowsiness, fatigue, agitation – CVS: bradycardia, syncope generally mild in severity, short-lived, related to titration (slowly!) caution in patients with asthma, CHOPD, cardiac conduction defects/clinically significant bradycardia

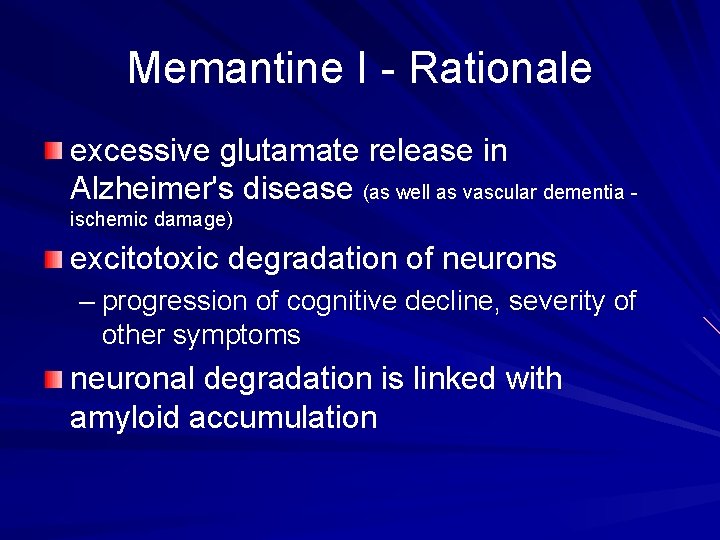
Memantine I - Rationale excessive glutamate release in Alzheimer's disease (as well as vascular dementia ischemic damage) excitotoxic degradation of neurons – progression of cognitive decline, severity of other symptoms neuronal degradation is linked with amyloid accumulation

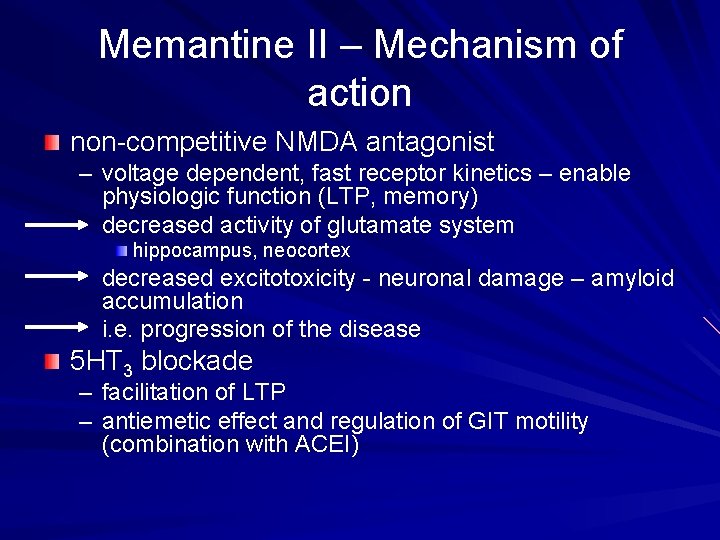
Memantine II – Mechanism of action non-competitive NMDA antagonist – voltage dependent, fast receptor kinetics – enable physiologic function (LTP, memory) decreased activity of glutamate system hippocampus, neocortex decreased excitotoxicity - neuronal damage – amyloid accumulation i. e. progression of the disease 5 HT 3 blockade – facilitation of LTP – antiemetic effect and regulation of GIT motility (combination with ACEI)

Memantine III – Clinical efficacy slower progression of vascular and Alzheimer dementia fast onset of action – 2 weeks improvement of cognitive functions, vigility, daily activities reduction of the need for the help of caregivers efficacy even in the moderate to severe disease stages x ACEI

Memantine IV Adverse events – generally well tolerated – higher than placebo: insomnia, dizziness, headache, hallucinations (NMDA antagonist – PCP) Pharmacokinetics – – renal excretion no extensive metabolization no cytochrome P 450 inhibition dosage 20 mg pro die in 2 doses start: 5 mg, titration: 5 mg per week

Treatment – important suggestions Seek and treat mild stages – current treatment modalities are more effective in mild AD Control comorbid conditions Help patients to stay at home as long as possible Work with the patient´s family

Other causes of dementia

Vascular dementia the second most common cause of dementia due to tissue damage (CVS) clinical manifestations - variable – focal neurological symptoms – cortical (cortical infarctions) / subcortical (lacunar infarctions, Binswanger´s disease) „pattern“ – sudden onset (often together with CVS accident) – may be stepwise progression (dropped from DSM IV) – may be patchy neuropsychological impairment (vs. global in AD; dropped from DSM IV) – relatively spared personality and insight Hachinski score – incorporate vascular risk and course features: designed to distinguish AD and VD; now under criticism Treatment: – control of the CVS risk (beta blockers and cognitive dysfunction)

Parkinson's disease „subcortical“ dementia degeneration of SN (also putamen, globus pallidus, caudate) loss of DA cells (also 5 HT) „ 3 M: movement, mentation, mood“ – rigidity, brady/hypokinesia – slow mental processing, executive and visuospatial abnormalities, 20 -30% full picture of dementia – depression ~ 40%

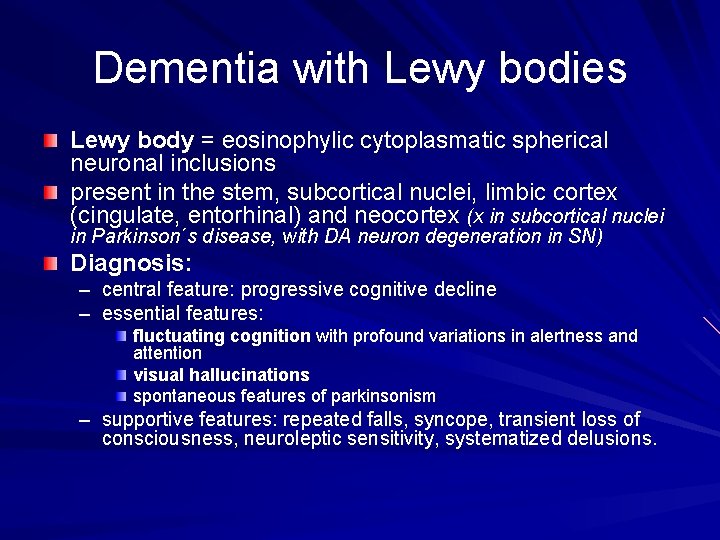
Dementia with Lewy bodies Lewy body = eosinophylic cytoplasmatic spherical neuronal inclusions present in the stem, subcortical nuclei, limbic cortex (cingulate, entorhinal) and neocortex (x in subcortical nuclei in Parkinson´s disease, with DA neuron degeneration in SN) Diagnosis: – – central feature: progressive cognitive decline essential features: fluctuating cognition with profound variations in alertness and attention visual hallucinations spontaneous features of parkinsonism – supportive features: repeated falls, syncope, transient loss of consciousness, neuroleptic sensitivity, systematized delusions.

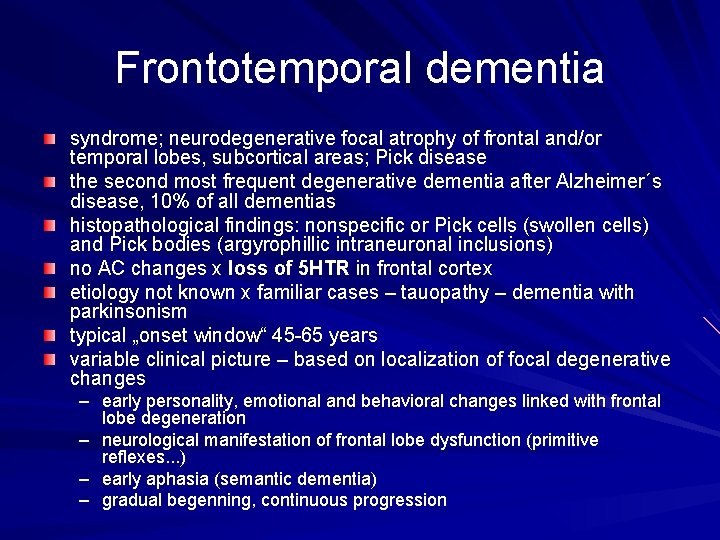
Frontotemporal dementia syndrome; neurodegenerative focal atrophy of frontal and/or temporal lobes, subcortical areas; Pick disease the second most frequent degenerative dementia after Alzheimer´s disease, 10% of all dementias histopathological findings: nonspecific or Pick cells (swollen cells) and Pick bodies (argyrophillic intraneuronal inclusions) no AC changes x loss of 5 HTR in frontal cortex etiology not known x familiar cases – tauopathy – dementia with parkinsonism typical „onset window“ 45 -65 years variable clinical picture – based on localization of focal degenerative changes – early personality, emotional and behavioral changes linked with frontal lobe degeneration – neurological manifestation of frontal lobe dysfunction (primitive reflexes. . . ) – early aphasia (semantic dementia) – gradual begenning, continuous progression

Creutzfeld-Jacob´s disease Etiology: prions Clinical manifestation: – – – rapid progression of dementia (lethal within 2 years) neurological symptoms terminal stage is typically manifested as akinetic and mute state – characteristic EEG: periodic spikes against a slow and low-voltage background No specific treatment

Huntington´s disease Etiology: autosomal dominant hereditary disease – neurodegeneration Clinical manifestation – dementia with characteristic movement disorder: choreiform involuntary movements of face, hands, shoulders No specific treatment

„Take home message“ Dementia is a common condition We can partly influence its course now Better chance to treat it in the mild, early stages Help patients to stay in their homes (with adequate care)

References : Waldinger R. J. : Psychiatry for medical students, Washington, DC : American Psychiatric Press, 1997 Kaplan HI, Sadock BJ, Grebb JA. : Kaplan and Sadock´s synopsis of psychiatry, Baltimore: Williams and Wilkins, 1997
 Tom kitwood enriched model of dementia care
Tom kitwood enriched model of dementia care Mood mse
Mood mse Tomáš garrigue masaryk prezentace
Tomáš garrigue masaryk prezentace Psychiatry in ethiopia
Psychiatry in ethiopia Neurosis vs psychosis
Neurosis vs psychosis Masaryk university library
Masaryk university library Biblografie
Biblografie Masaryk memorial cancer institute
Masaryk memorial cancer institute Core psychiatry
Core psychiatry Geriatric psychiatry definition
Geriatric psychiatry definition Daniel chen md
Daniel chen md Is muni cz
Is muni cz Masaryk university medical faculty
Masaryk university medical faculty Internal medicine shelf exam passing score
Internal medicine shelf exam passing score Pediprn
Pediprn Ascaris lumbricoides ova
Ascaris lumbricoides ova National network of child psychiatry access programs
National network of child psychiatry access programs Forensic psychiatry vs forensic psychology
Forensic psychiatry vs forensic psychology Criminal psychology means
Criminal psychology means Global initiative on psychiatry
Global initiative on psychiatry Tomáš garrigue masaryk prezentace
Tomáš garrigue masaryk prezentace Radical psychiatry sociology
Radical psychiatry sociology Community geriatric psychiatry
Community geriatric psychiatry European psychiatry
European psychiatry Asclepiades father of psychiatry
Asclepiades father of psychiatry Mse abstract thinking
Mse abstract thinking Addiction expert witnesses
Addiction expert witnesses Erp sysetm
Erp sysetm Ceitec masaryk university
Ceitec masaryk university Tomtom go 910 update
Tomtom go 910 update What does the bible symbolize in the devil and tom walker
What does the bible symbolize in the devil and tom walker Dep training modules
Dep training modules Avt dep
Avt dep Cpu dep
Cpu dep Dep environmental education grants
Dep environmental education grants Dep horticulture
Dep horticulture Nstoria
Nstoria Dep hkps
Dep hkps Jini surrogate architecture
Jini surrogate architecture Giô dép
Giô dép Dep
Dep How to remove personnel in lis
How to remove personnel in lis