Delivery of InsulinLike Growth Factor I to the






























- Slides: 48

“Delivery of Insulin-Like Growth Factor -I to the Rat Brain and Spinal cord along Olfactory and Trigeminal Pathways Following Intranasal Administration. ” Thorne, R. G. , Pronk, G. J. , Padmanabhan, V. , and Frey II, W. H. Presented by Mattia Migliore October 13, 2005

Introduction: § § Neurotrophic factors are proteins that help promote the development, survival, repair, and proliferation of neurons. Term derived from the Greek “trophimos, ” which means nourishing or nutritious. Usually hydrophilic, basic proteins with molecular weights ranging from 5 -30 k. Da. Prototypic neurotrophic factor is nerve growth factor (NGF), but more than 20 different neurotrophic factors have been identified.

Introduction (Cont. ): § § Neurotrophic factors have been shown to exert their pharmacological effects at concentrations of only nanomolar to femtomolar ranges. Potential curative treatments for neurodegenerative disorders including Parkinson’s disease, Alzheimer’s disease, and to reverse the neurological damage caused by strokes and/or head injuries.

Limitations to therapeutic use of neurotrophic factors for the treatment of neurodegenerative diseases: Exogenously administered neurotrophic factors cannot reach their site of action because they do not cross the blood-brain barrier.

Limitations to therapeutic use of neurotrophic factors for the treatment of neurodegenerative diseases (cont. ): § § Also, they have very short half-lives due to their rapid clearance and degradation by plasma hydrolytic enzymes. Currently, the only ways to deliver a neutrophic factor into the brain are by either injecting the peptide intraparenchymally, or by administering the peptide into the cerebroventricular (IVC) cavity.

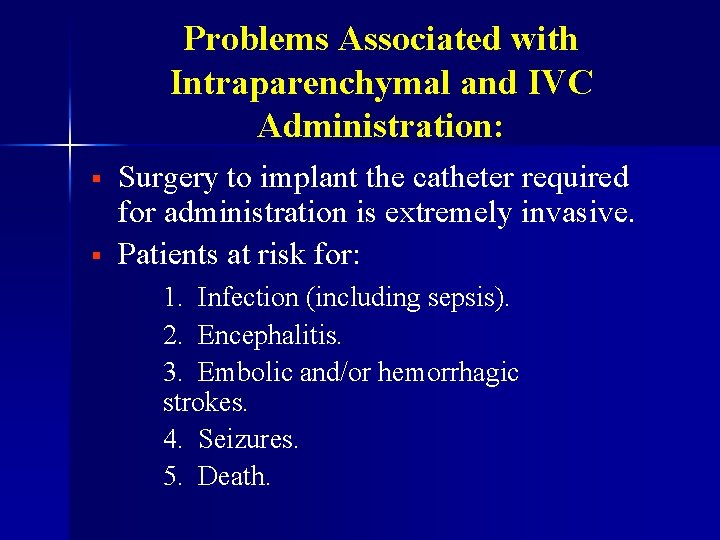
Problems Associated with Intraparenchymal and IVC Administration: § § Surgery to implant the catheter required for administration is extremely invasive. Patients at risk for: 1. Infection (including sepsis). 2. Encephalitis. 3. Embolic and/or hemorrhagic strokes. 4. Seizures. 5. Death.

Why can’t neurotrophic factors cross the blood-brain barrier?

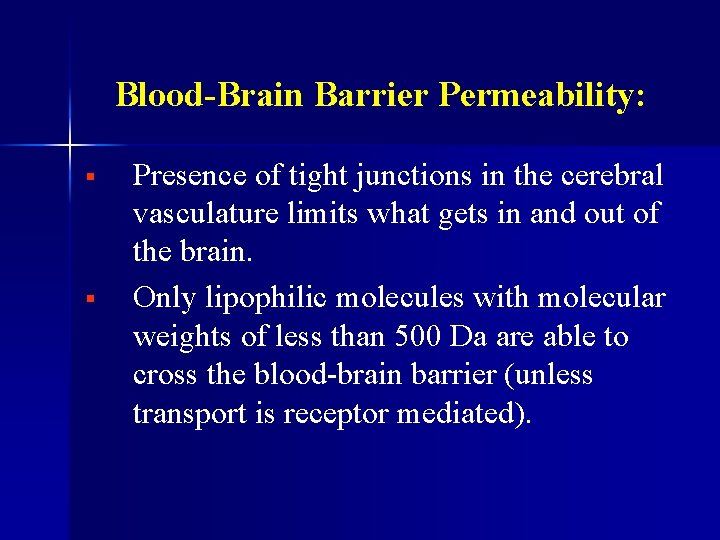
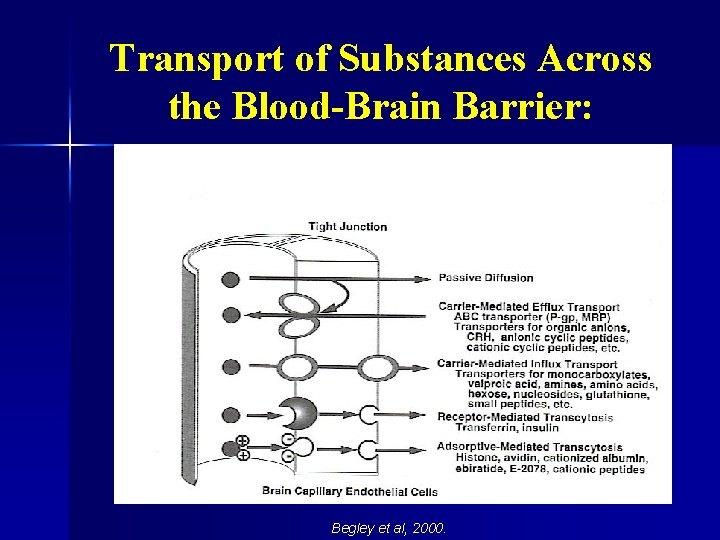
Blood-Brain Barrier Permeability: § § Presence of tight junctions in the cerebral vasculature limits what gets in and out of the brain. Only lipophilic molecules with molecular weights of less than 500 Da are able to cross the blood-brain barrier (unless transport is receptor mediated).

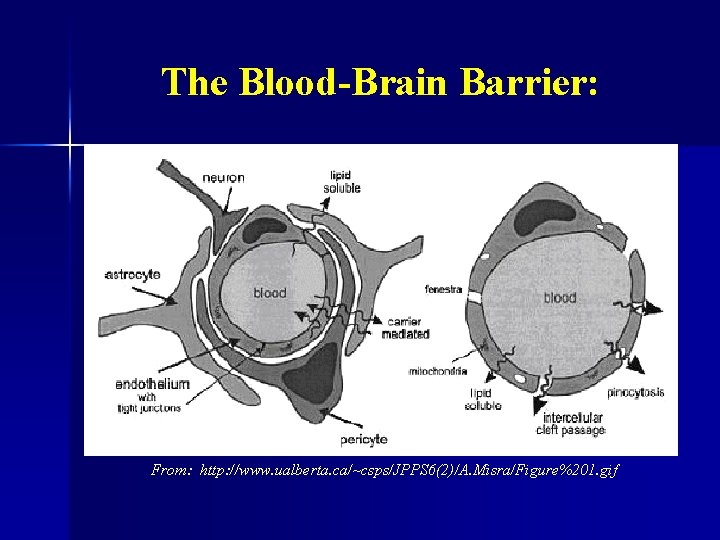
The Blood-Brain Barrier: From: http: //www. ualberta. ca/~csps/JPPS 6(2)/A. Misra/Figure%201. gif

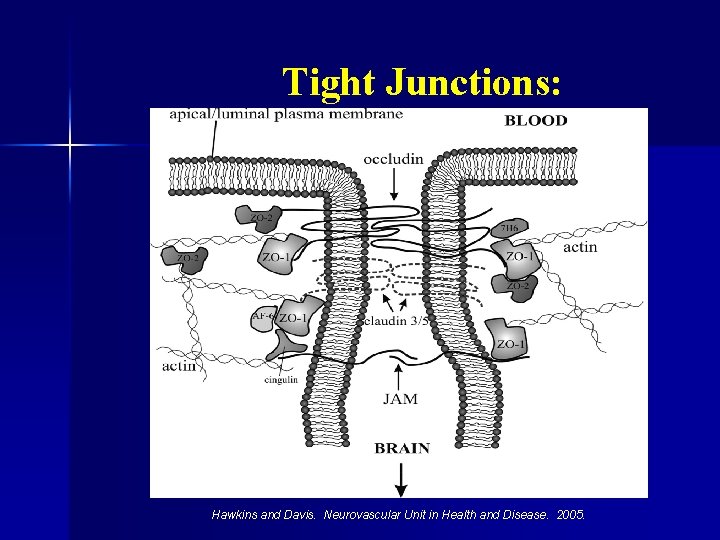
Tight Junctions: Hawkins and Davis. Neurovascular Unit in Health and Disease. 2005.

Transport of Substances Across the Blood-Brain Barrier: Begley et al, 2000.

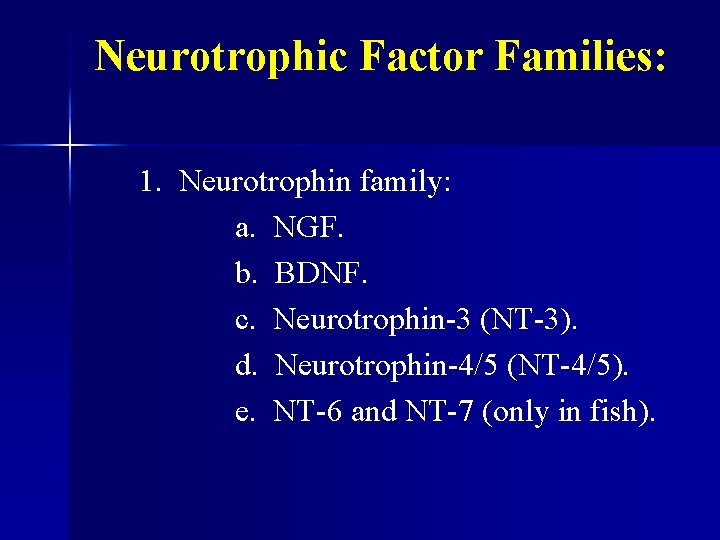
Neurotrophic Factor Families: 1. Neurotrophin family: a. NGF. b. BDNF. c. Neurotrophin-3 (NT-3). d. Neurotrophin-4/5 (NT-4/5). e. NT-6 and NT-7 (only in fish).

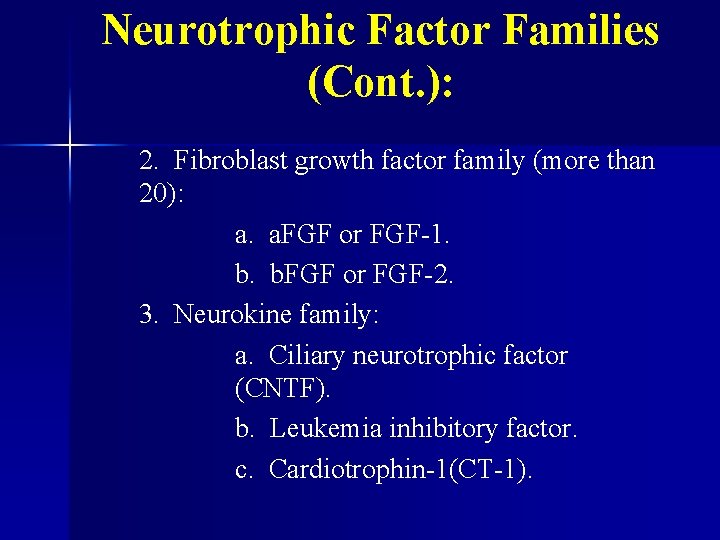
Neurotrophic Factor Families (Cont. ): 2. Fibroblast growth factor family (more than 20): a. a. FGF or FGF-1. b. b. FGF or FGF-2. 3. Neurokine family: a. Ciliary neurotrophic factor (CNTF). b. Leukemia inhibitory factor. c. Cardiotrophin-1(CT-1).

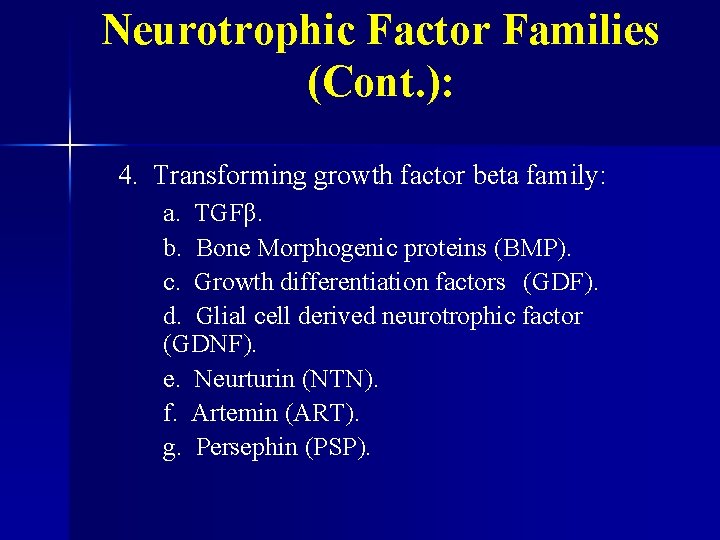
Neurotrophic Factor Families (Cont. ): 4. Transforming growth factor beta family: a. TGFβ. b. Bone Morphogenic proteins (BMP). c. Growth differentiation factors (GDF). d. Glial cell derived neurotrophic factor (GDNF). e. Neurturin (NTN). f. Artemin (ART). g. Persephin (PSP).

Neurotrophic Factor Families (Cont. ): 5. Epidermal growth factor family: a. EGF. b. Transforming growth factor α. 6. Platelet derived growth factors. 7. Activity-dependent growth factor (ADNF). 8. Insulin-like growth factor family: a. IGF-1. b. IGF-2.

Insulin-Like Growth Factors: § Functions: 1. 2. 3. 4. § § Cell growth. Differentiation. Migration. Survival. IGF-I is a single polypeptide chain with a 70 amino acid sequence, and a molecular weight of 7. 65 KDa. IGF-I has similar 3 -D structure to proinsulin.

Insulin-like Growth Factors (Cont. ): § § IGF-II is a 67 amino acid peptide with structural homology to IGF-I is believed to promote neuronal survival and/or regeneration, and is a much more potent growth factor than IGF-2. IGF-1 is the main mediator of growth hormone’s actions. In humans, the IGF-I gene has been mapped to chromosome 12.

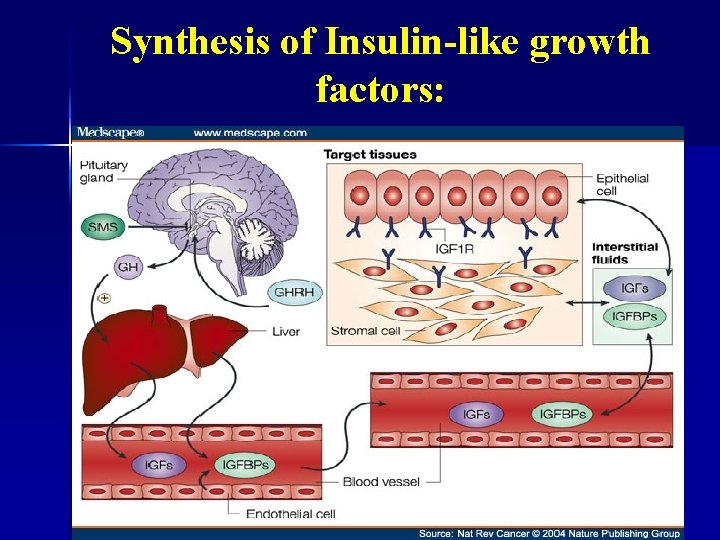
Endocrine Vs. Paracrine Release. § Endocrine release: IGF-I is primarily made in the liver in response to growth hormone stimulation. § IGF-II synthesis, on the other hand, appears to be independent from the influences of GH. Paracrine release: IGF-I can also be synthesized locally in response to various trophic hormones (seemingly independently from GH). §

Synthesis of Insulin-like growth factors:

Transport of Insulin like growth factors: n n n IGFs circulate in the blood bound to IGF-binding proteins (IGFBPs). Functions of IGFBPs are to transport and to store IGFs. There are 6 different types of IGFBPs and they all bind to both IGFs. IGFBPs regulate the interaction of IGFs with their receptors. They are regulated through phosphorylation, proteolysis, and through association with cell components.

IGFs receptors: § § § Found in all tissues. Members of the tyrosine kinase receptor family. Consist of 2 alpha and 2 beta subunits joined by disulfide cross bridges. Insulin receptors can also bind IGF-1 and IGF-2. IGF-1 receptor can also bind IGF-2 and insulin. IGF-2 receptor can’t bind insulin, but it can bind IGF-1.


Hypothesis: “Intranasally delivered IGF-1 can bypass the blood-brain barrier via olfactory and trigeminal associated extracellular pathways to rapidly elicit biological effects at multiple sites within the brain and spinal cord. ”

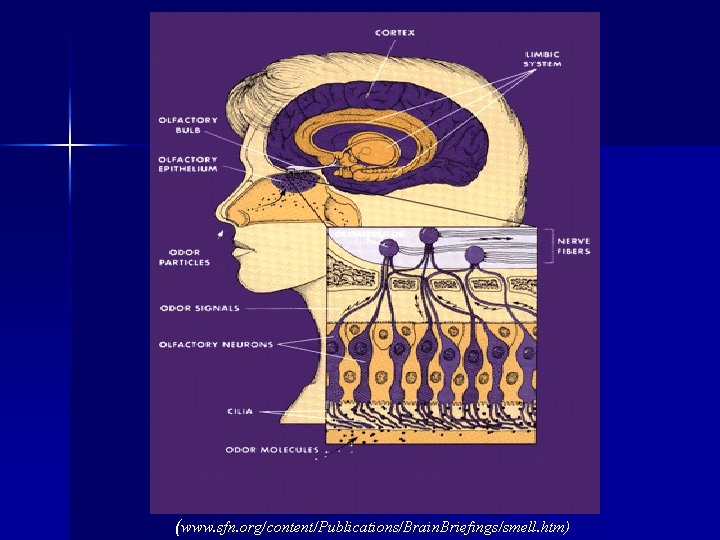
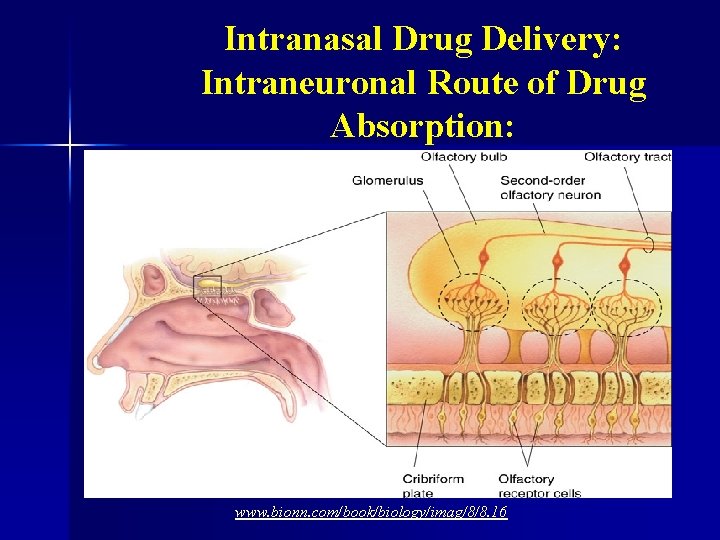
Intranasal Administration: § Intranasal drug delivery takes advantage of the presence of an incomplete blood brain barrier in the olfactory epithelium (Graff and Pollack, 2005). § The olfactory nerves are able to completely bypass the blood brain barrier, and drugs that can be taken up by these neurons can be transported directly into the brain (Graff and Pollack, 2005).

(www. sfn. org/content/Publications/Brain. Briefings/smell. htm)

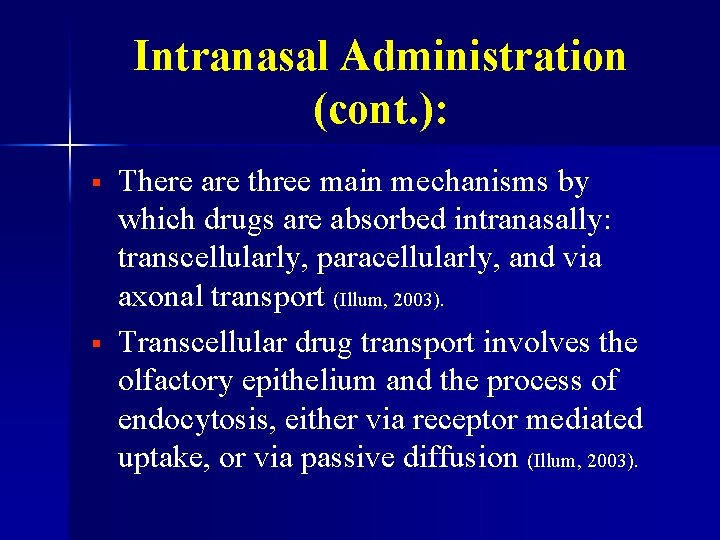
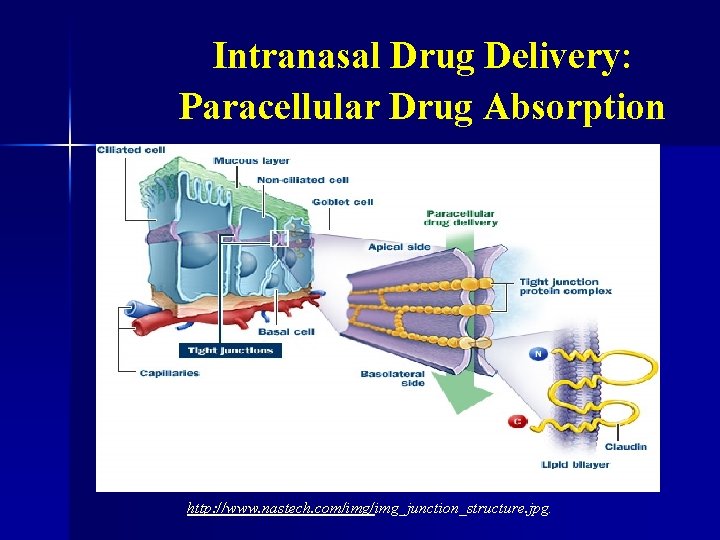
Intranasal Administration (cont. ): § § There are three main mechanisms by which drugs are absorbed intranasally: transcellularly, paracellularly, and via axonal transport (Illum, 2003). Transcellular drug transport involves the olfactory epithelium and the process of endocytosis, either via receptor mediated uptake, or via passive diffusion (Illum, 2003).

Intranasal Drug Delivery: Paracellular Drug Absorption http: //www. nastech. com/img_junction_structure. jpg.

Intranasal Drug Delivery: Intraneuronal Route of Drug Absorption: www. bionn. com/book/biology/imag/8/8. 16

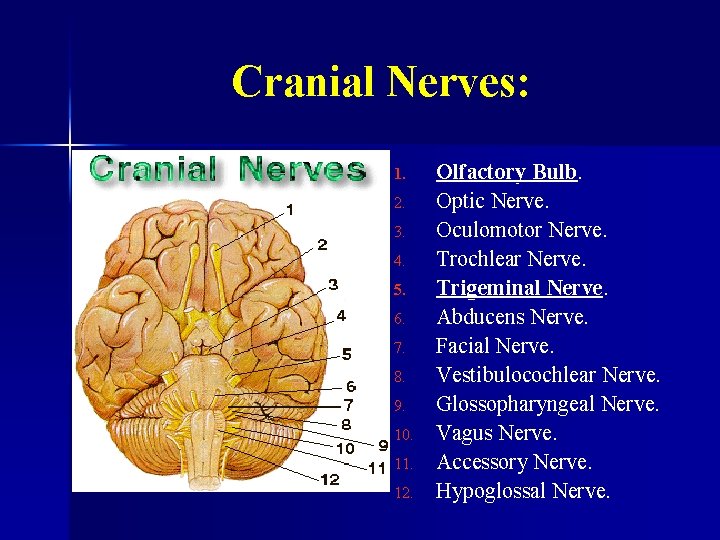
Cranial Nerves: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Olfactory Bulb. Optic Nerve. Oculomotor Nerve. Trochlear Nerve. Trigeminal Nerve. Abducens Nerve. Facial Nerve. Vestibulocochlear Nerve. Glossopharyngeal Nerve. Vagus Nerve. Accessory Nerve. Hypoglossal Nerve.

Materials and Methods: § § § SD rats w/ weights of 180 g-305 g used. Recombinant human IGF-1 used (96% homology w/ rat IGF-1). [125 I]-IGF-1 (w/ less than 1% unbound iodine) was ultrafiltrated x 2 prior to each experiment to remove unbound iodine.

Materials and Methods (cont. ): § § § Activity in cpm/µl was determined by a gamma counter in tripicate. Biotinylated IGF-1(2. 3 mol biotin/mole IGF-1) was used. Catheters were implanted into the abdominal aorta and/or the femoral vein for blood sampling.

Materials and Methods (cont. ): § § IN administration: Rats were anesthetized and placed in a supine position. Total of 50 μl total of [125 I]-IGF-1 solution (5. 0 nmol, 143 mcg IGF/kg of body weight, specific activity of 3. 5 Ci/mmol) was administered via pipette by drops alternating between nostrils over 18. 5 min. IV administration: Rats placed in supine position and 500 µl [125 I]-IGF-1 (0. 011 nmol IGF -1, 0. 33 mcg/Kg, 1900 Ci/mmol) injected via femoral vein over 1 -2 min.

Materials and Methods (cont. ): § § Blood sampled Q 5 min from abdominal aorta in both groups. Animals transcardially perfused w/ saline then fixed w/ mixture of glutaraldehyde and paraformaldehyde approximately 30 min after start of administration.

Materials and Methods (cont. ): § § § One group of IN administered rats was allowed to recover from anesthesia and blood was sampled at 0. 5, 2, 6, and 24 hrs after the start of administration. One group of IN administered rats (52 µl, 8. 4 nmol, 290 mcg/Kg, specific activity= 1. 6 Ci/mmol) were used to sample blood and CSF. Post fixation, superficial and deep cervical lymph nodes were dissected as well as dura mater from the brain and blood vessels.

Materials and Methods (cont. ): § § § Fixed brain was serial sectioned (1 mm) w/ a coronal precision rat brain matrix. Other areas of the brain were taken via corers (12 mm) using a micropunch technique. Autoradiography was performed on two groups: High specific activity IN (2100 Ci/mmol) and Low specific activity IN (4. 0 Ci/mmol).

Materials and Methods (cont. ): § § Immunohistochemistry and Immunoblotting was performed on two groups of IN administration: 60μl biotinylated IGF-I or control (PBS). Animals were sacrificed and fixed after 24 -28 min after the start of the administration. Then brain tissue was sectioned and later incubated w/ a 1: 1000 dilution of a murine monoclonal HRP-labeled antiphosphotyrosine antibody to visualize tyrosine phosphorylated proteins (by using metal enhanced DAB).

Materials and Methods (cont. ): § Immunobloating was performed using a monoclonal antibody against phosphotyrosine.











Conclusion: § Intranasal administration of therapeutic proteins for the treatment of neurodegenerative disorders may overcome the limitations on the use of neurotrophic factors by providing a safer, more effective method of drug delivery to the brain.
 What are the component of accenture delivery suite
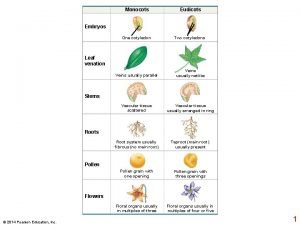
What are the component of accenture delivery suite Primary growth and secondary growth in plants
Primary growth and secondary growth in plants Geometric growth population
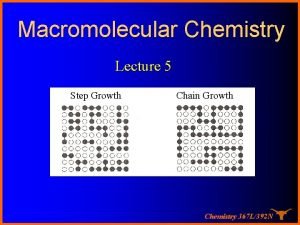
Geometric growth population Growthchain
Growthchain Neoclassical growth theory vs. endogenous growth theory
Neoclassical growth theory vs. endogenous growth theory Primary growth and secondary growth in plants
Primary growth and secondary growth in plants Organic growth vs inorganic growth
Organic growth vs inorganic growth Plant growth index
Plant growth index Chapter 35 plant structure growth and development
Chapter 35 plant structure growth and development Growth factors mitosis
Growth factors mitosis Testrovax ingredients
Testrovax ingredients Form factor and crest factor
Form factor and crest factor What is factored form
What is factored form Non researchable questions
Non researchable questions Quiz 7-2 factoring by gcf
Quiz 7-2 factoring by gcf Common factors of 36 and 48
Common factors of 36 and 48 Factor out the greatest common factor
Factor out the greatest common factor Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Phản ứng thế ankan
Phản ứng thế ankan Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Lp html
Lp html Sơ đồ cơ thể người
Sơ đồ cơ thể người Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Thang điểm glasgow
Thang điểm glasgow ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Cái miệng nó xinh thế
Cái miệng nó xinh thế Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết V cc
V cc Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chúa yêu trần thế
Chúa yêu trần thế Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Diễn thế sinh thái là
Diễn thế sinh thái là