Degeneration and Regeneration of Neurons Adapted from internet





- Slides: 26

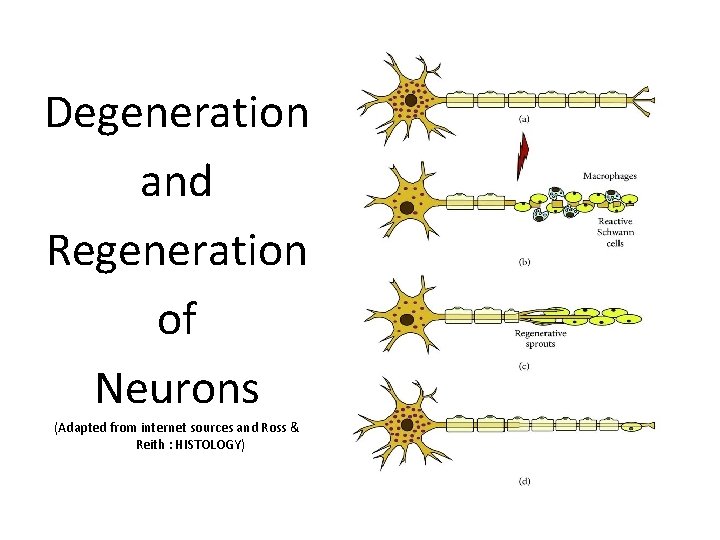
Degeneration and Regeneration of Neurons (Adapted from internet sources and Ross & Reith : HISTOLOGY)

HISTORY • Wallerian degeneration is named after Augustus Volney Waller. • Waller experimented on frogs in 1850, by severing glossopharyngeal and hypoglossal nerves. • He then observed the distal nerves from the site of injury, which were separated from their cell bodies in the brain stem. • Waller described the disintegration of myelin, which he referred to as "medulla", into separate particles of various sizes. • The degenerating axons formed droplets that could be stained, thus allowing for studies of the course of individual nerve fibres.

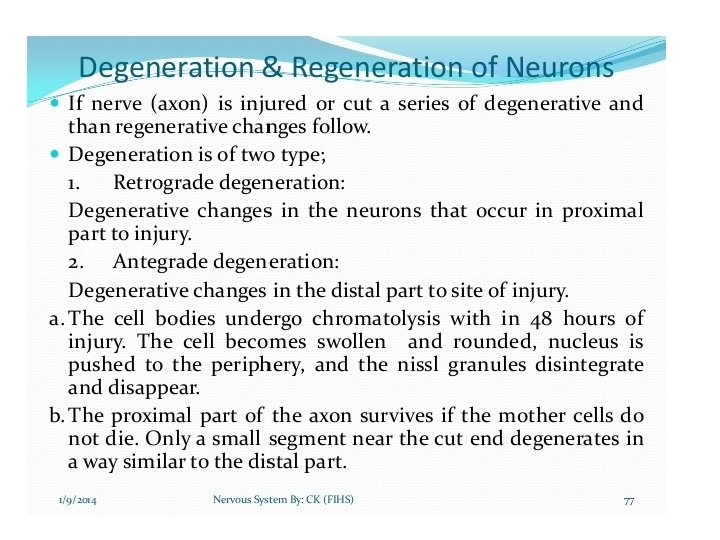
• Wallerian degeneration is an active process of degeneration that results when a nerve fiber is cut or crushed and the part of the axon distal to the injury (i. e. farther from the neuron's cell body) degenerates. • A related process of dying back or retrograde degeneration known as 'Wallerian-like degeneration' occurs in many neurodegenerative diseases, especially those where axonal transport is impaired such as ALS and Alzheimer's disease. • Primary culture studies suggest that a failure to deliver sufficient quantities of the essential axonal protein NMNAT 2 is a key initiating event.

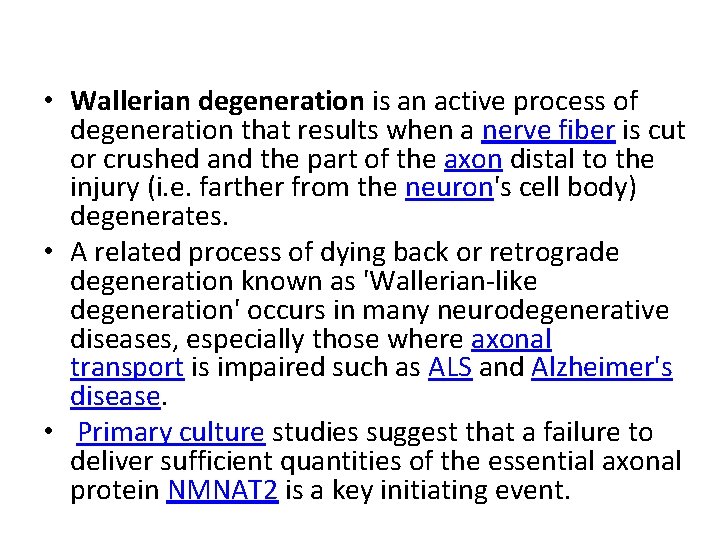
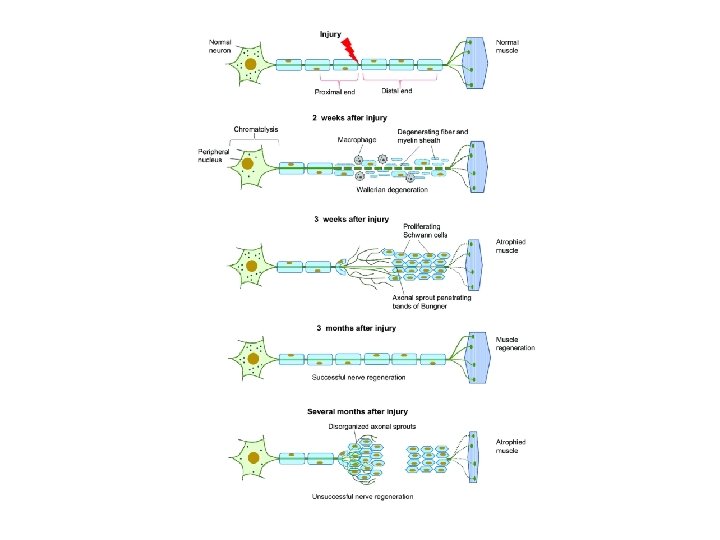
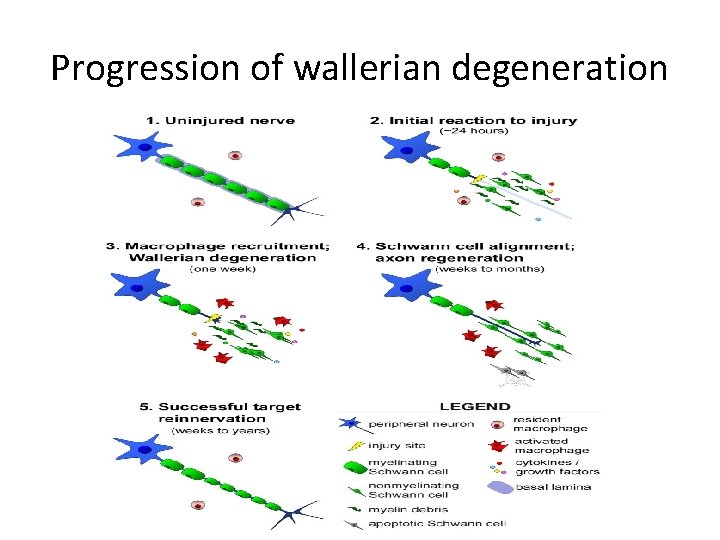
• Wallerian degeneration occurs after axonal injury in both the peripheral nervous system (PNS) and central nervous system (CNS). • It occurs in the section of the axon distal to the site of injury and usually begins within 24– 36 hours of a lesion. • Prior to degeneration, the distal section of the axon tends to remain electrically excitable. • After injury, the axonal skeleton disintegrates, and the axonal membrane breaks apart. • Axonal degeneration is followed by degradation of the myelin sheath and infiltration by macrophages. • The macrophages, accompanied by Schwann cells, serve to clear the debris from the degeneration.


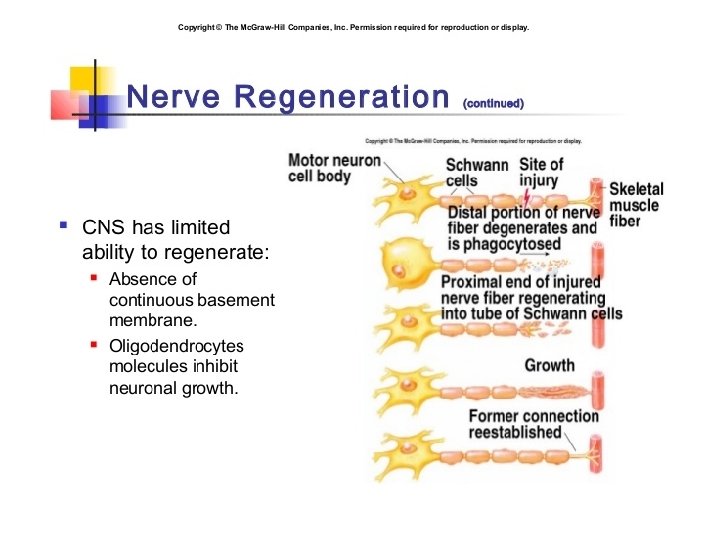
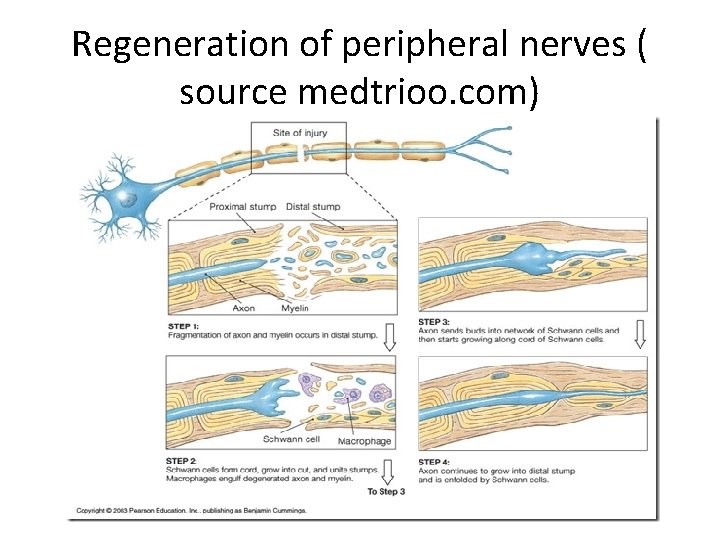
• Schwann cells respond to loss of axons by extrusion of their myelin sheaths, downregulation of myelin genes, dedifferentiation and proliferation. • They finally align in tubes (Büngner bands) and express surface molecules that guide regenerating fibers. • Within 4 days of the injury, the distal end of the portion of the nerve fiber proximal to the lesion sends out sprouts towards those tubes and these sprouts are attracted by growth factors produced by Schwann cells in the tubes. • If a sprout reaches the tube, it grows into it and advances about 1 mm per day, eventually reaching and reinnervating the target tissue. • If the sprouts cannot reach the tube, for instance because the gap is too wide or scar tissue has formed, surgery can help to guide the sprouts into the tubes

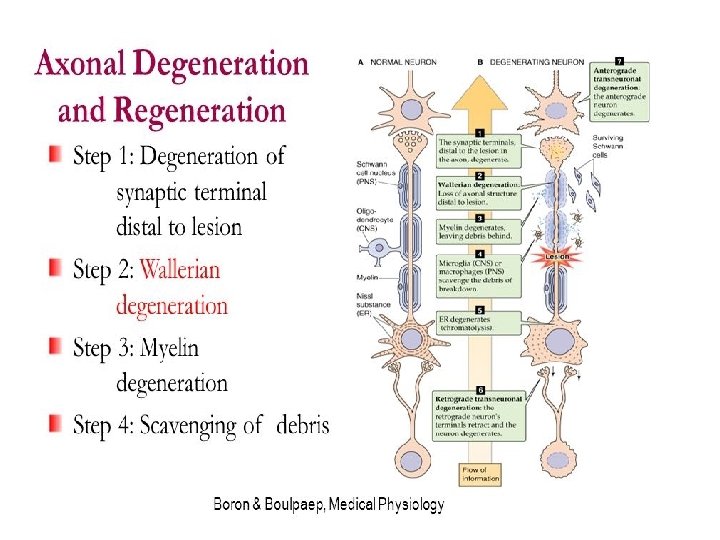
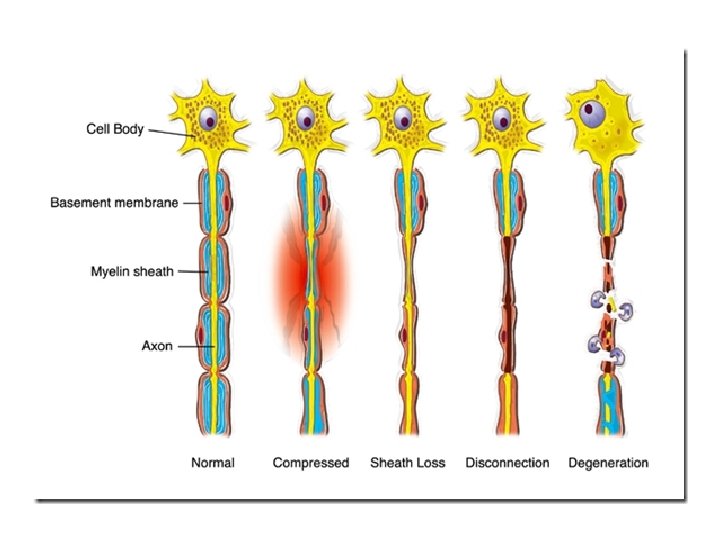
Progression of wallerian degeneration

Cellular response to nerve injury source DOI: 10. 2147/MDER. S 59124

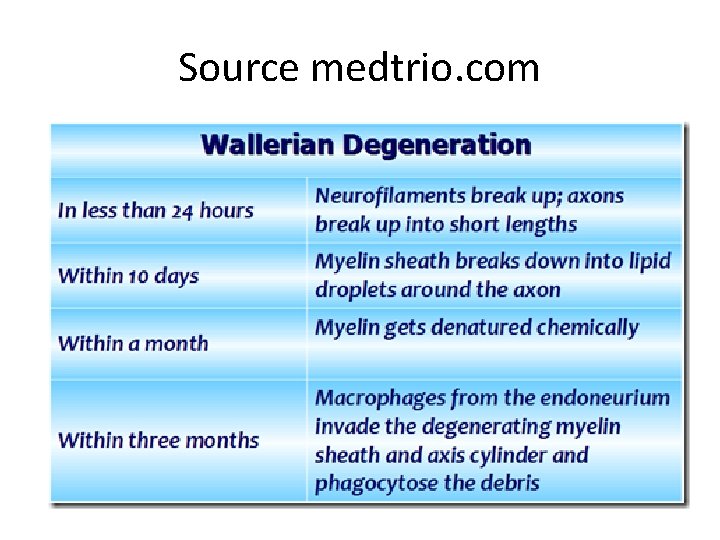
Source medtrio. com

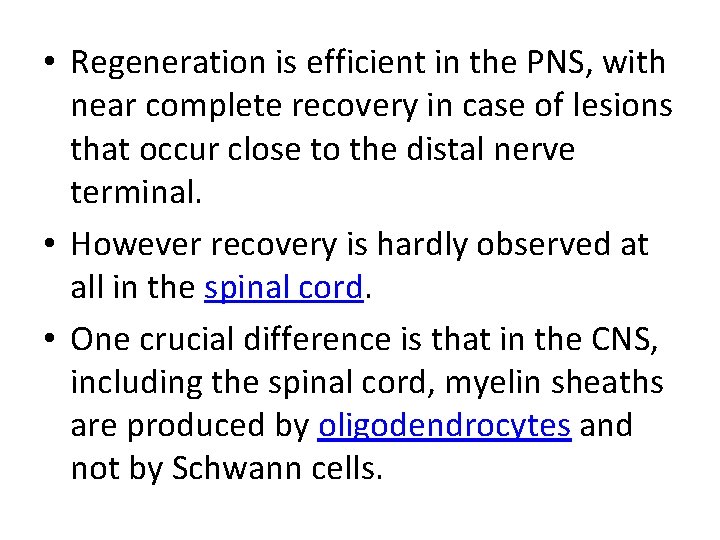
• Regeneration is efficient in the PNS, with near complete recovery in case of lesions that occur close to the distal nerve terminal. • However recovery is hardly observed at all in the spinal cord. • One crucial difference is that in the CNS, including the spinal cord, myelin sheaths are produced by oligodendrocytes and not by Schwann cells.

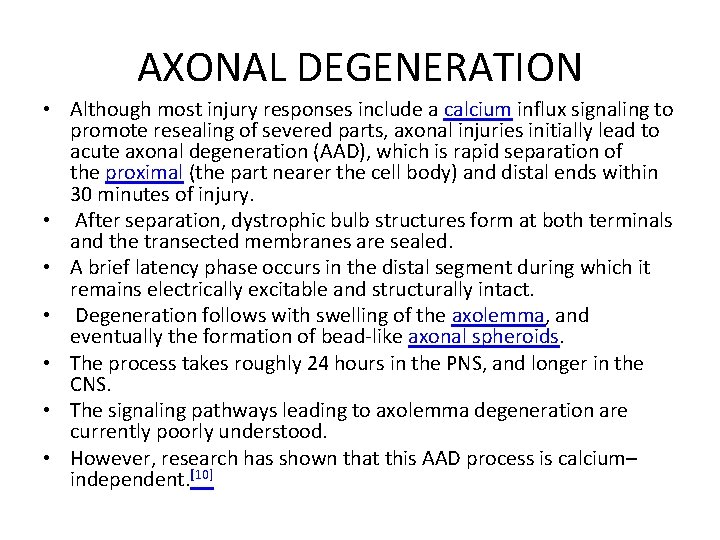
AXONAL DEGENERATION • Although most injury responses include a calcium influx signaling to promote resealing of severed parts, axonal injuries initially lead to acute axonal degeneration (AAD), which is rapid separation of the proximal (the part nearer the cell body) and distal ends within 30 minutes of injury. • After separation, dystrophic bulb structures form at both terminals and the transected membranes are sealed. • A brief latency phase occurs in the distal segment during which it remains electrically excitable and structurally intact. • Degeneration follows with swelling of the axolemma, and eventually the formation of bead-like axonal spheroids. • The process takes roughly 24 hours in the PNS, and longer in the CNS. • The signaling pathways leading to axolemma degeneration are currently poorly understood. • However, research has shown that this AAD process is calcium– independent. [10]


• Granular disintegration of the axonal cytoskeleton and inner organelles occurs after axolemma degradation. • Early changes include accumulation of mitochondria in the paranodal regions at the site of injury. Endoplasmic reticulum degrades and mitochondria swell up and eventually disintegrate. • The depolymerization of microtubules occurs and is soon followed by degradation of the neurofilaments and other cytoskeleton components. • The disintegration is dependent on Ubiquitin and Calpain proteases (caused by influx of calcium ion), suggesting that axonal degeneration is an active process and not a passive one as previously misunderstood. [11] • Thus the axon undergoes complete fragmentation. • The rate of degradation is dependent on the type of injury and is also slower in the CNS than in the PNS. • Another factor that affects degradation rate is the diameter of the axon: larger axons require a longer time for the cytoskeleton to degrade and thus take a longer time to degenerate.


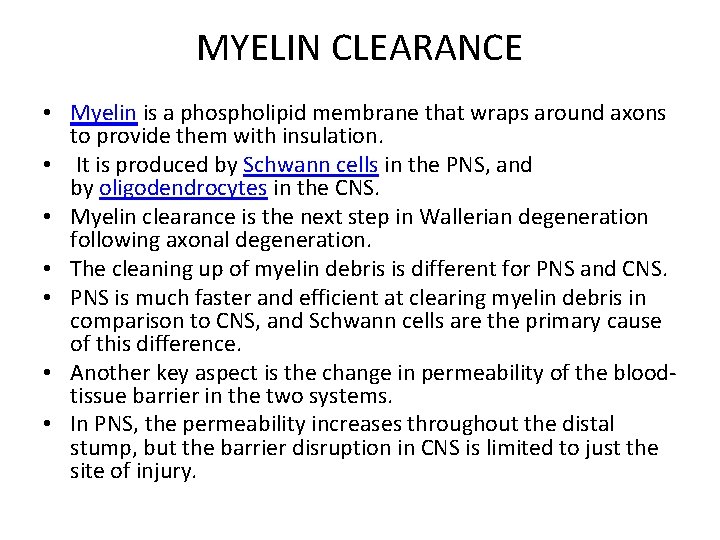
MYELIN CLEARANCE • Myelin is a phospholipid membrane that wraps around axons to provide them with insulation. • It is produced by Schwann cells in the PNS, and by oligodendrocytes in the CNS. • Myelin clearance is the next step in Wallerian degeneration following axonal degeneration. • The cleaning up of myelin debris is different for PNS and CNS. • PNS is much faster and efficient at clearing myelin debris in comparison to CNS, and Schwann cells are the primary cause of this difference. • Another key aspect is the change in permeability of the bloodtissue barrier in the two systems. • In PNS, the permeability increases throughout the distal stump, but the barrier disruption in CNS is limited to just the site of injury.

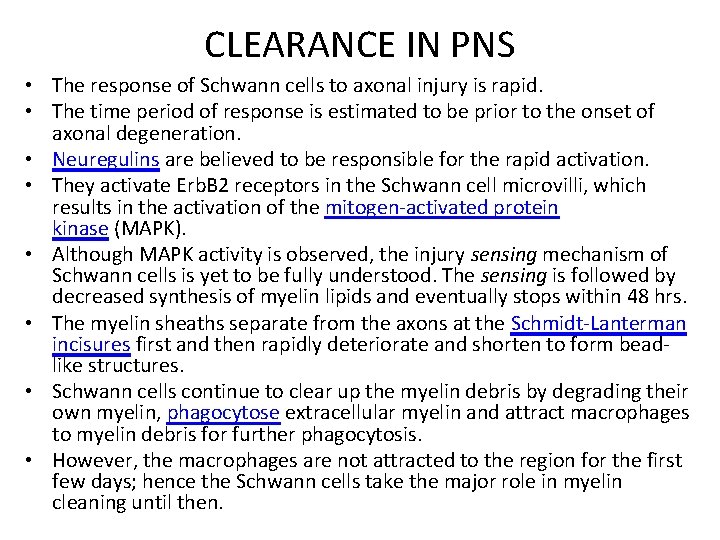
CLEARANCE IN PNS • The response of Schwann cells to axonal injury is rapid. • The time period of response is estimated to be prior to the onset of axonal degeneration. • Neuregulins are believed to be responsible for the rapid activation. • They activate Erb. B 2 receptors in the Schwann cell microvilli, which results in the activation of the mitogen-activated protein kinase (MAPK). • Although MAPK activity is observed, the injury sensing mechanism of Schwann cells is yet to be fully understood. The sensing is followed by decreased synthesis of myelin lipids and eventually stops within 48 hrs. • The myelin sheaths separate from the axons at the Schmidt-Lanterman incisures first and then rapidly deteriorate and shorten to form beadlike structures. • Schwann cells continue to clear up the myelin debris by degrading their own myelin, phagocytose extracellular myelin and attract macrophages to myelin debris for further phagocytosis. • However, the macrophages are not attracted to the region for the first few days; hence the Schwann cells take the major role in myelin cleaning until then.

• Schwann cells have been observed to recruit macrophages by release of cytokines and chemokines after sensing of axonal injury. • The recruitment of macrophages helps improve the clearing rate of myelin debris. • The resident macrophages present in the nerves release further chemokines and cytokines to attract further macrophages. The degenerating nerve also produce macrophage chemotactic molecules. • Another source of macrophage recruitment factors is serum. Delayed macrophage recruitment was observed in B-cell deficient mice lacking serum antibodies. • These signaling molecules together cause an influx of macrophages, which peaks during the third week after injury. • While Schwann cells mediate the initial stage of myelin debris clean up, macrophages come in to finish the job. • Macrophages are facilitated by opsonins, which label debris for removal. • The 3 major groups found in serum include complement, pentraxins, and antibodies. • However, only complement has shown to help in myelin debris phagocytosis.

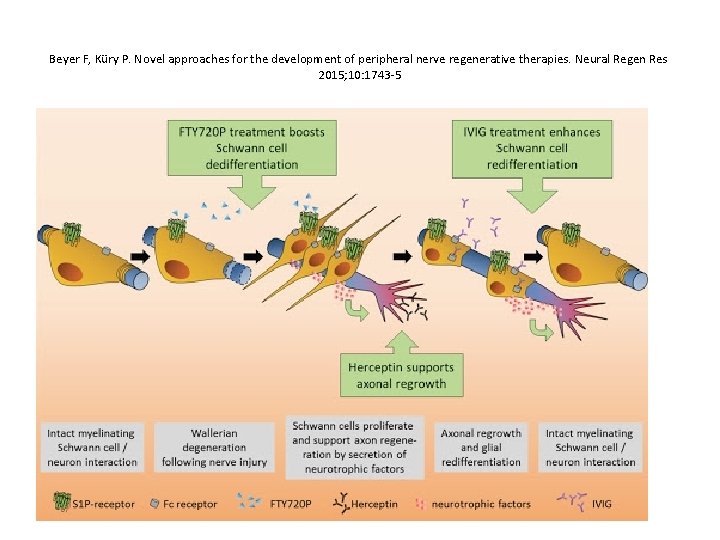
Beyer F, Küry P. Novel approaches for the development of peripheral nerve regenerative therapies. Neural Regen Res 2015; 10: 1743 -5

CLEARANCE IN CNS • • • In comparison to Schwann cells, oligodendrocytes require axon signals to survive. In their developmental stages, oligodendrocytes that fail to make contact to axon and receive axon signals undergo apoptosis. upon injury oligodendrocytes either undergo programmed cell death or enter a state of rest. Therefore, unlike Schwann cells, oligodendrocytes fail to clean up the myelin sheaths and their debris. In experiments conducted on rats, myelin sheaths were found for up to 22 months. Therefore, CNS rates of myelin sheath clearance are very slow and could possibly be the cause for hindrance in the regeneration capabilities of the CNS axons as no growth factors are available to attract the proximal axons. Another feature that results eventually is Glial scar formation. This further hinders chances for regeneration and reinnervation. Oligodendrocytes fail to recruit macrophages for debris removal. Macrophage entry in general into CNS site of injury is very slow. In contrast to PNS, Microglia play a vital role in CNS wallerian degeneration. However, their recruitment is slower in comparison to macrophage recruitment in PNS by approximately 3 days. The rate of clearance is very slow among microglia in comparison to macrophages. Possible source for variations in clearance rates could include lack of opsonin activity around microglia, and the lack of increased permeability in the blood–brain barrier. The decreased permeability could further hinder macrophage infiltration to the site of injury. These findings have suggested that the delay in Wallerian degeneration in CNS in comparison to PNS is caused not due to a delay in axonal degeneration, but rather is due to the difference in clearance rates of myelin in CNS and PNS.

Source: dollyhost. com


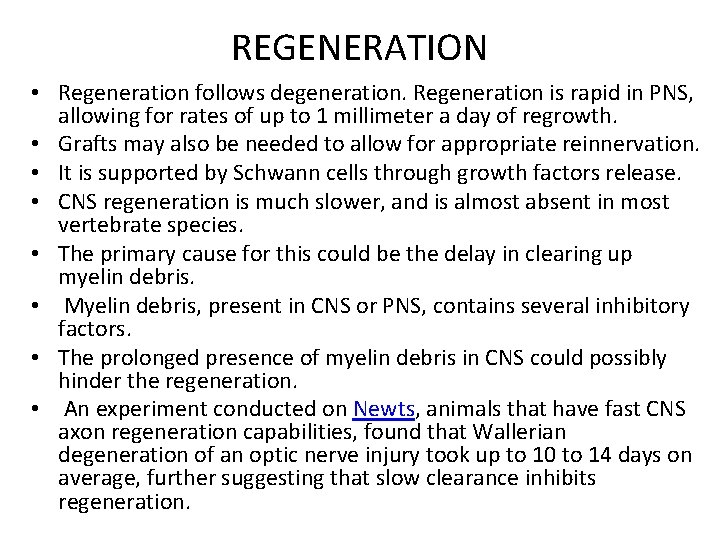
REGENERATION • Regeneration follows degeneration. Regeneration is rapid in PNS, allowing for rates of up to 1 millimeter a day of regrowth. • Grafts may also be needed to allow for appropriate reinnervation. • It is supported by Schwann cells through growth factors release. • CNS regeneration is much slower, and is almost absent in most vertebrate species. • The primary cause for this could be the delay in clearing up myelin debris. • Myelin debris, present in CNS or PNS, contains several inhibitory factors. • The prolonged presence of myelin debris in CNS could possibly hinder the regeneration. • An experiment conducted on Newts, animals that have fast CNS axon regeneration capabilities, found that Wallerian degeneration of an optic nerve injury took up to 10 to 14 days on average, further suggesting that slow clearance inhibits regeneration.

Regeneration of peripheral nerves ( source medtrioo. com)

CHROMATOLYSIS • Chromatolysis is the dissolution of the Nissl bodies in the cell body of a neuron. • It is an induced response of the cell usually triggered by axotomy, ischemia, toxicity to the cell, cell exhaustion, virus infections, and hibernation in lower vertebrates. • Neuronal recovery through regeneration can occur after chromatolysis, but most often it is a precursor of apoptosis. • The event of chromatolysis is also characterized by a prominent migration of the nucleus towards the periphery of the cell and an increase in the size of the nucleolus, nucleus, and cell body. • The term "chromatolysis" was initially used in the 1940 s to describe the observed form of cell death characterized by the gradual disintegration of nuclear components; a process which is now called apoptosis. • Chromatolysis is still used as a term to distinguish the particular apoptotic process in the neuronal cells, where Nissl substance disintegrates.

TYPES OF CHROMATOLYSIS • Central chromatolysis is the most common form of chromatolysis and is characterized by the loss or dispersion of the Nissl bodies starting near the nucleus at the center of the neuron, and then extending peripherally towards the plasma membrane. • Also characteristic of central chromatolysis is the displacement of the nucleus towards the periphery of the perikaryon. • Other cellular changes are observed during the process of the central chromatolysis. • The process of Nissl dissolution is less apparent toward periphery of the cell body of the neuron, where normal-looking Nissl bodies may be present. • Hyperplasia of neurofilaments is frequently observed, however the extent varies. • The number of autophagic vacuoles and lysosomal structures often increase during central chromatolysis. • Changes can also occur in other organelles such as the Golgi apparatus and neurotubules. • However, the exact significance of these changes is currently unknown.

PERIPHERAL CHROMATOLYSIS • Peripheral chromatolysis is much less common, but has been reported to occur after axotomy and ischemia in certain species. • Peripheral chromatolysis is essentially the reverse of central chromatolysis, in which the disintegration of Nissl bodies is initiated at the periphery of the neuron and extends inwards towards the nucleus of the cell. • Peripheral chromatolysis has been observed to occur in lithium-induced chromatolysis and it could be useful in investigating and countering the hypothesis that waves of enzymatic activity always progress from the perinuclear area, or the area situated around the nucleus, to the peripheral of the cell.
 Adapted from the internet
Adapted from the internet Gas turbine with regeneration
Gas turbine with regeneration Was the london docklands regeneration a success
Was the london docklands regeneration a success Objectives of artificial regeneration
Objectives of artificial regeneration Brayton cycle with regeneration problems
Brayton cycle with regeneration problems Fiat doblo dpf regeneration procedure
Fiat doblo dpf regeneration procedure Planarian regeneration
Planarian regeneration What is regeneration
What is regeneration Classification of gas turbine
Classification of gas turbine Cellules ciliées régénération
Cellules ciliées régénération Guided tissue regenration
Guided tissue regenration Nottingham regeneration
Nottingham regeneration Nasir farooq
Nasir farooq Wallerian degeneration
Wallerian degeneration Subacute combined degeneration
Subacute combined degeneration Cs amplifier with source degeneration
Cs amplifier with source degeneration Terrien marginal degeneration
Terrien marginal degeneration Cornea verticillata
Cornea verticillata Magdalena karadža
Magdalena karadža Cobblestone degeneration
Cobblestone degeneration Muscular degeneration
Muscular degeneration Tobacco dust appearance
Tobacco dust appearance Ruffini corpuscles
Ruffini corpuscles Mount classification of dental caries
Mount classification of dental caries First second and third order neurons
First second and third order neurons Somatic motor association area
Somatic motor association area Stretch reflex
Stretch reflex