Deflagration Safety Study Of Mixtures of Hydrogen and




















- Slides: 43

Deflagration Safety Study Of Mixtures of Hydrogen and Natural Gas In A Semi-Open Space Erik Merilo Mark Groethe Poulter Laboratory Menlo Park, CA, USA © 2007 SRI International

Outline • Introduction • Previous Research • Test Facility • Gas Mixtures • Measurements • Test Procedure • Results • Summary © 2007 SRI International 2

Introduction • In the transition to a hydrogen economy it is likely that hydrogen will be used or stored in close proximity to other flammable fuels and gases • Accidents can occur that result in the release of two or more fuels –Home garage –Repair facility –Parking structure –Tunnel with different types of vehicles • Hydrogen and natural gas can mix and form a hazard © 2007 SRI International 3

Hydrogen-Methane Mixtures and Hydrogen. Hydrocarbon Previous Research • Research has been conducted on the combustion of hydrogen-methane mixtures and other hydrogenhydrocarbon mixtures • Laminar burning velocity has been experimentally measured for different gas compositions and equivalency ratios (F) • Computational simulations have been performed to model laminar burning velocity for hydrogen methane mixtures over a wide range of conditions © 2007 SRI International 4

Hydrogen-Methane Mixtures and Hydrogen. Hydrocarbon Previous Research • Methane-rich mixtures – Addition of hydrogen enhances the combustion • Hydrogen-rich fuel mixtures – Addition of methane has an inhibiting effect on the hydrogen combustion • For hydrogen-methane mixtures, the laminar burning velocity decreases with the increase of the fuel’s methane mole fraction • The inhibitive effect of methane addition and the enhancement caused by hydrogen addition are the result of H radical concentration and the associated chemical effects © 2007 SRI International 5

Hydrogen-Methane Mixtures and Hydrogen. Hydrocarbon Previous Research • Majority of work has focused on laminar burning velocity • For real-world accident scenarios and explosions, flame propagation is almost always turbulent • Computer simulations use the laminar burning velocity to model turbulent combustion in the laminar flamelet approach • To validate and increase the accuracy of computer models it is also important to have experimental data for medium and large scale deflagrations of flammable gas mixtures • The flame speeds and generated overpressures need to be measured in order to improve the accuracy of computer models and to improve risk assessment capabilities © 2007 SRI International 6

Introduction • Five medium-scale semi-open space deflagration experiments have been conducted with stoichiometric mixtures of hydrogen, natural gas, and air – Hydrogen mole fraction varied from: – Natural gas mole fraction varied from: 1. 000 to 0. 897 0. 000 to 0. 103 – Previous test was performed with natural gas mole fraction of 1. 000 • Unconfined deflagrations performed in a semi-open space – Semi-open space: Confined by a frame and tent • Primary interest in these experiments was the measurement of the blast overpressure – Tests investigate gas mixtures where small amounts of natural gas were © 2007 SRI International 7

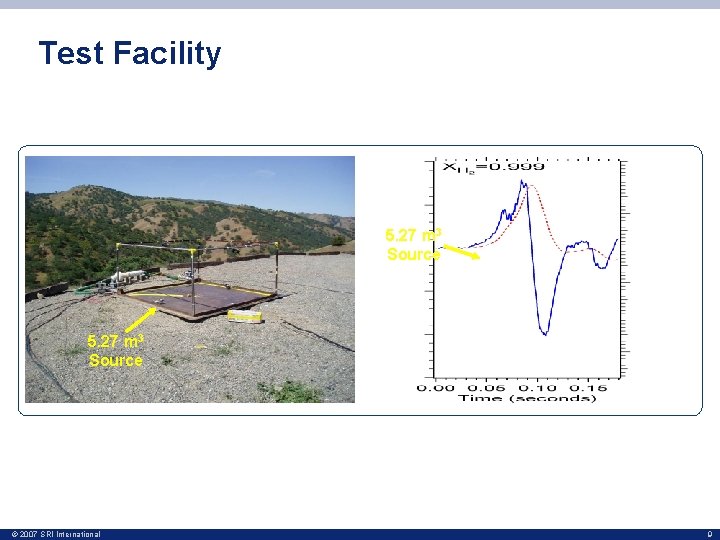
Test Facility • 5. 27 m³ facility has a welded steel frame welded to a steel floor • The facility dimensions are 2. 24 m square by 1. 05 m tall • The mixture volume was created by covering the frame with a sheet of high density polyethylene (HDPE) film having a thickness of 0. 008 mm • HDPE film was glued to the 5. 27 m³ facility © 2007 SRI International 8

Test Facility 5. 27 m 3 Source © 2007 SRI International 9

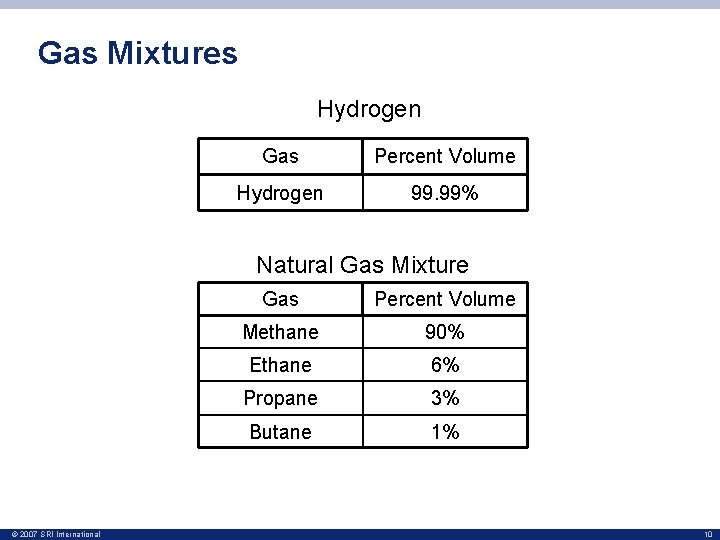
Gas Mixtures Hydrogen Gas Percent Volume Hydrogen 99. 99% Natural Gas Mixture © 2007 SRI International Gas Percent Volume Methane 90% Ethane 6% Propane 3% Butane 1% 10

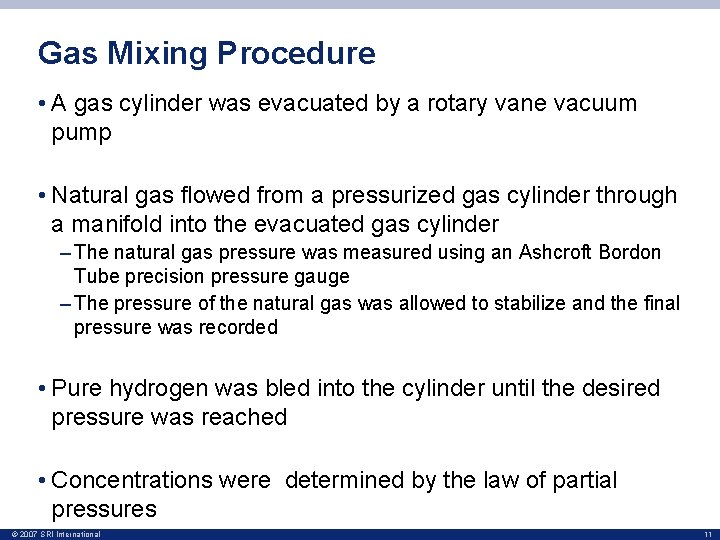
Gas Mixing Procedure • A gas cylinder was evacuated by a rotary vane vacuum pump • Natural gas flowed from a pressurized gas cylinder through a manifold into the evacuated gas cylinder – The natural gas pressure was measured using an Ashcroft Bordon Tube precision pressure gauge – The pressure of the natural gas was allowed to stabilize and the final pressure was recorded • Pure hydrogen was bled into the cylinder until the desired pressure was reached • Concentrations were determined by the law of partial pressures © 2007 SRI International 11

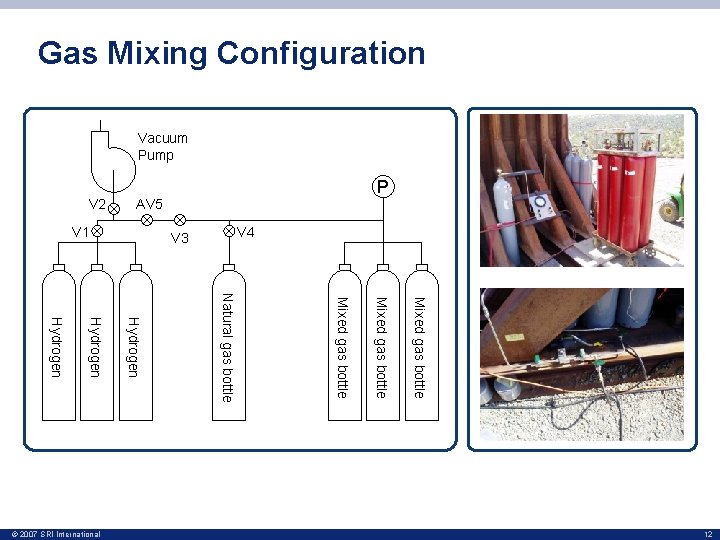
Gas Mixing Configuration Vacuum Pump Mixed gas bottle Natural gas bottle Hydrogen 12 © 2007 SRI International AV 5 V 2 V 4 V 3 V 1 P

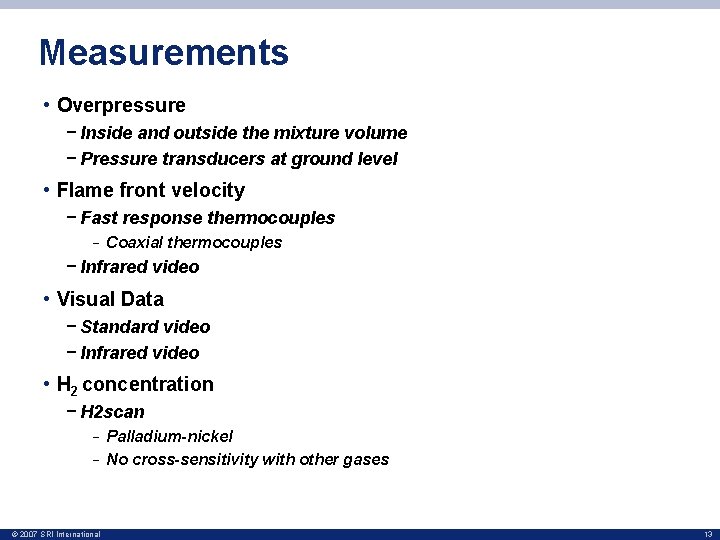
Measurements • Overpressure − Inside and outside the mixture volume − Pressure transducers at ground level • Flame front velocity − Fast response thermocouples − Coaxial thermocouples − Infrared video • Visual Data − Standard video − Infrared video • H 2 concentration − H 2 scan − − © 2007 SRI International Palladium-nickel No cross-sensitivity with other gases 13

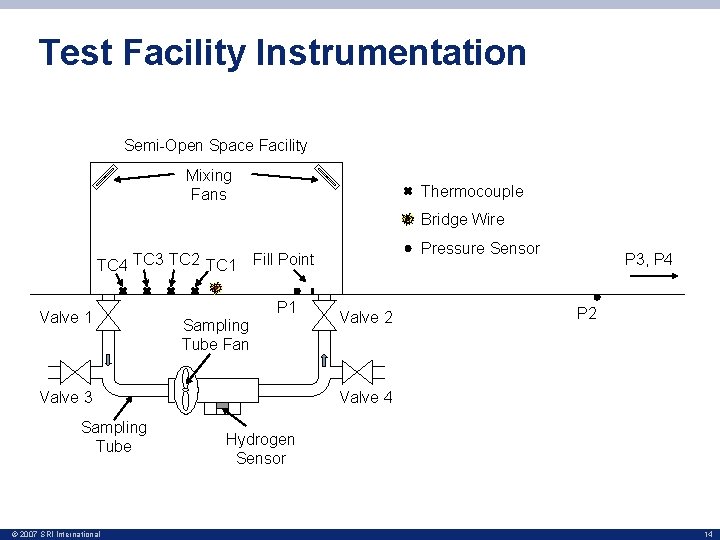
Test Facility Instrumentation Semi-Open Space Facility Mixing Fans Thermocouple Bridge Wire TC 4 TC 3 TC 2 TC 1 Valve 1 P 1 Sampling Tube Fan Valve 3 Sampling Tube © 2007 SRI International Pressure Sensor Fill Point Valve 2 P 3, P 4 P 2 Valve 4 Hydrogen Sensor 14

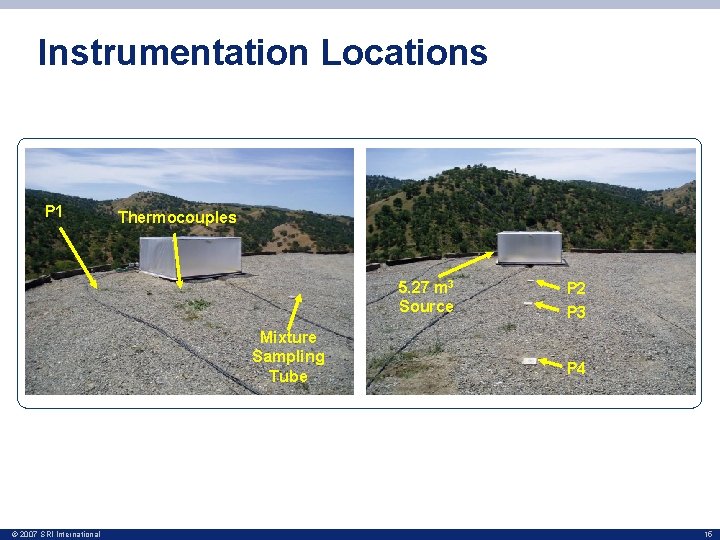
Instrumentation Locations P 1 Thermocouples 5. 27 m 3 Source Mixture Sampling Tube © 2007 SRI International P 2 P 3 P 4 15

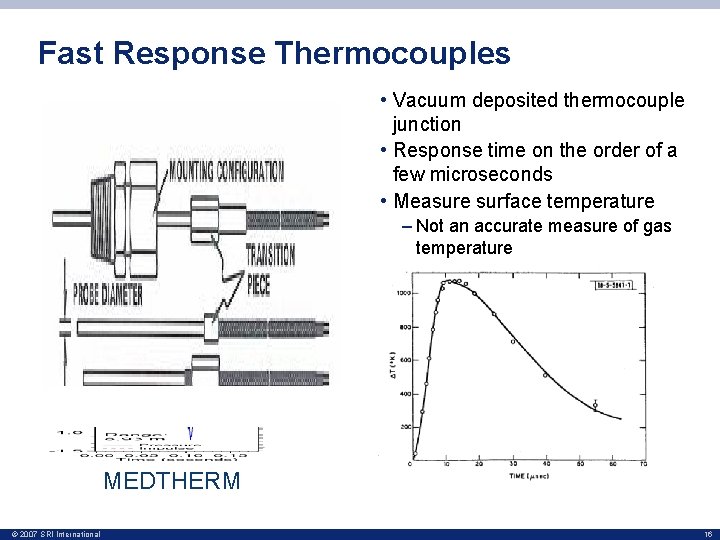
Fast Response Thermocouples • Vacuum deposited thermocouple junction • Response time on the order of a few microseconds • Measure surface temperature – Not an accurate measure of gas temperature – Used for TOA measurements MEDTHERM © 2007 SRI International 16

Fast Response Thermocouples Junction Flame Propagation Direction © 2007 SRI International 17

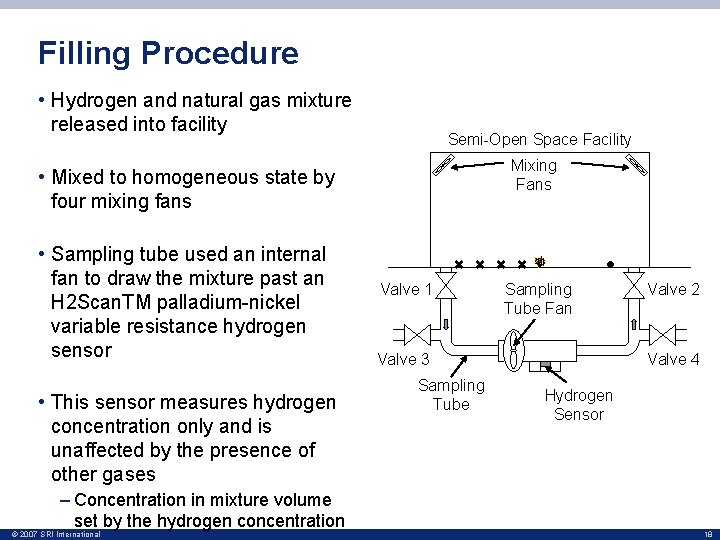
Filling Procedure • Hydrogen and natural gas mixture released into facility Semi-Open Space Facility Mixing Fans • Mixed to homogeneous state by four mixing fans • Sampling tube used an internal fan to draw the mixture past an H 2 Scan. TM palladium-nickel variable resistance hydrogen sensor • This sensor measures hydrogen concentration only and is unaffected by the presence of other gases – Concentration in mixture volume set by the hydrogen concentration © 2007 SRI International Valve 1 Sampling Tube Fan Valve 3 Sampling Tube Valve 2 Valve 4 Hydrogen Sensor 18

Fuel Mixture Mole Fractions Energy (MJ) Test XH 2 XNG F 1 1. 000 0. 000 1. 01 15. 12 2 0. 999 0. 001 15. 81 3 0. 990 0. 010 1. 00 15. 93 4 0. 949 0. 051 1. 00 15. 60 5 0. 897 0. 103 1. 02 16. 36 © 2007 SRI International 19

Ignition Procedure • When the hydrogen concentration reached the desired level the hydrogen-natural gas release valve was turned off and the fuel-air mixture was mixed for two minutes • The internal mixing fans were turned off and the mixture sampling tube was closed 30 seconds prior to ignition • The mixture was ignited at the bottom center of the enclosure with a spark created by a standard Du. Pont bridgewire connected to a capacitive discharge unit (CDU) • The total energy available from the CDU was about 40 J © 2007 SRI International 20

Standard and Infrared Video Frames Standard XH 2=0. 999 ~33 ms ~67 ms ~100 ms IR © 2007 SRI International 21

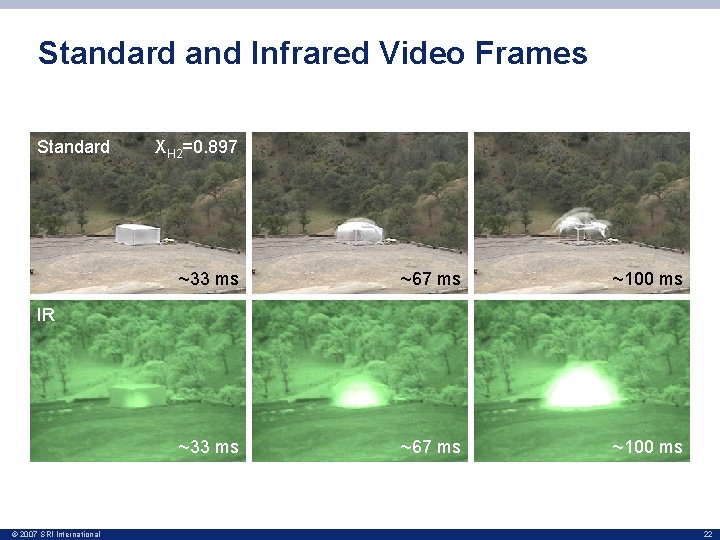
Standard and Infrared Video Frames Standard XH 2=0. 897 ~33 ms ~67 ms ~100 ms IR © 2007 SRI International 22

Infrared Video Frames XH 2=0. 999 IR ~33 ms ~67 ms ~100 ms XH 2=0. 897 IR © 2007 SRI International 23

IR Video Measurements • Only two frames of the advancing flame front were captured for most of the tests –Due to the velocity of the flame and the duration of the camera exposures • The velocity was calculated by averaging the distance the hemispheric flame traveled between the two frames • For Test 5, XH 2 = 0. 897, the flame speed was slow enough to allow three frames of the advancing flame front to be captured at ranges that were similar to thermocouple measurement points © 2007 SRI International 24

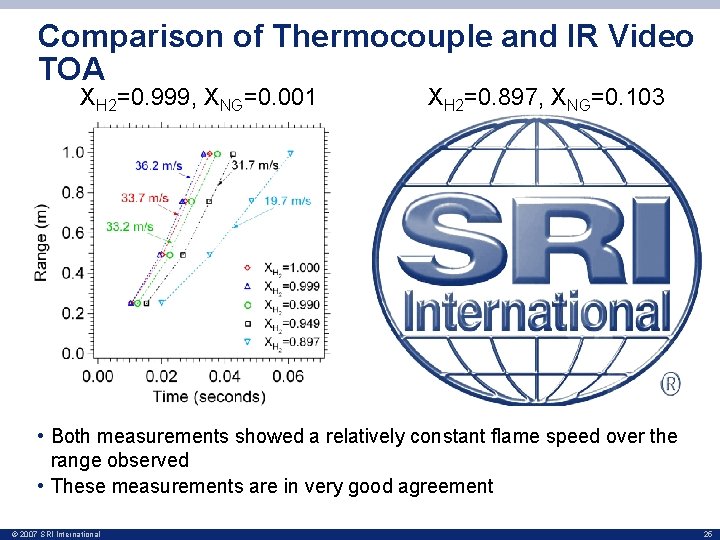
Comparison of Thermocouple and IR Video TOA XH 2=0. 999, XNG=0. 001 XH 2=0. 897, XNG=0. 103 • Both measurements showed a relatively constant flame speed over the range observed • These measurements are in very good agreement © 2007 SRI International 25

Comparison of Thermocouple and IR Video TOA • The flame front TOAs were measured over different ranges from the ignition source through use of thermocouples and IR video – Thermocouples – IR video frames 0. 25 m to 0. 99 m ~0. 1 m to 2. 5 m • Good agreement between the two measurements – Indicates that fast response thermocouples can be used to accurately measure flame propagation • The thermocouple data is more accurate because – Shorter response Time – Higher resolution – Locations are known precisely © 2007 SRI International 26

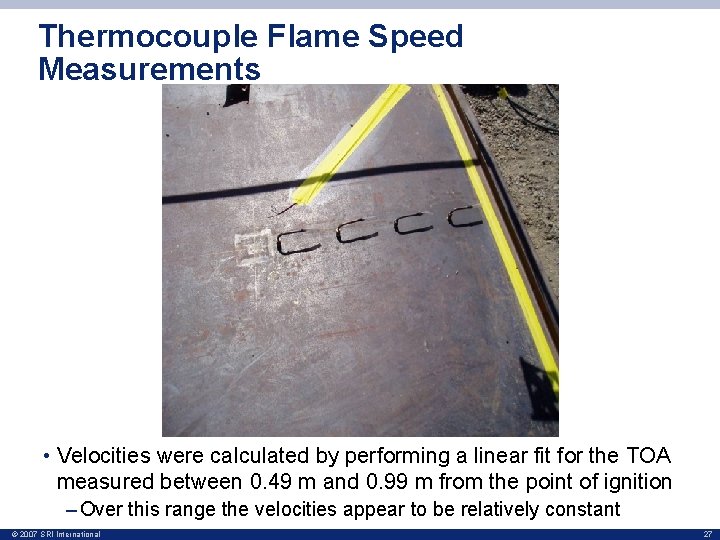
Thermocouple Flame Speed Measurements • Velocities were calculated by performing a linear fit for the TOA measured between 0. 49 m and 0. 99 m from the point of ignition – Over this range the velocities appear to be relatively constant © 2007 SRI International 27

Thermocouple Flame Speed Measurements © 2007 SRI International 28

Flame Speed © 2007 SRI International 29

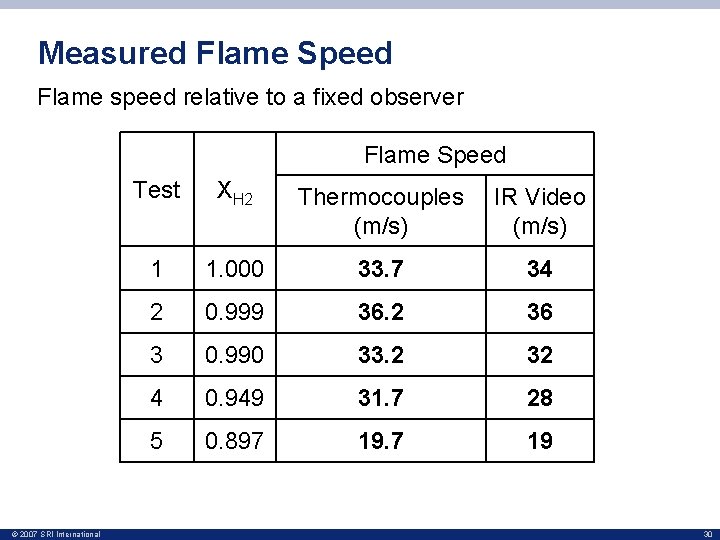
Measured Flame Speed Flame speed relative to a fixed observer Flame Speed © 2007 SRI International Test XH 2 Thermocouples (m/s) IR Video (m/s) 1 1. 000 33. 7 34 2 0. 999 36. 2 36 3 0. 990 33. 2 32 4 0. 949 31. 7 28 5 0. 897 19 30

Flame Speed and Burning Velocity Approximate Burning Velocity* (m/s) Mf Test XH 2 Flame Speed (m/s) 1 1. 000 33. 7 4. 8 0. 084 2 0. 999 36. 2 5. 2 0. 090 3 0. 990 33. 2 4. 7 0. 084 4 0. 949 31. 7 4. 5 0. 083 5 0. 897 19. 7 2. 8 0. 053 Burning Velocity: Flame front velocity relative to the unburned gas Mf: Mach Number relative to a fixed observer *Expansion ratio of 7 has been assumed © 2007 SRI International 31

Flame Speed • The highest flame speed fuel mixture with XH 2 = 0. 999 – Measured flame speed – Apparent Mach number 36. 2 m/s 0. 090 • Velocity measured in a test with pure hydrogen was 33. 7 m/s • For the gas mixtures with XH 2 ≤ 0. 990 the flame speed decreased with the increase in the natural gas mole fraction • For the test with XH 2 = 0. 897 the flame speed decreased to 19. 7 m/s and the apparent Mach number decreased to 0. 053 • In a previous test with a stoichiometric natural gas and air mixture (9. 5% vol. natural gas), XH 2 = 0. 000, a flame speed of 7. 5 m/s was measured at a range of 0. 8 m to 1. 0 m from the ignition point – Indicating a continuing downward trend © 2007 SRI International 32

Overpressure and Impulse • The average peak overpressures measured inside the mixture volume – 2. 60 k. Pa for XH 2 = 1. 000 – 1. 14 k. Pa for XH 2 = 0. 897 • The average peak overpressure measured inside the mixture volume for a stoichiometric natural gas and air mixture – < 0. 5 k. Pa for XH 2 = 0. 000 © 2007 SRI International 33

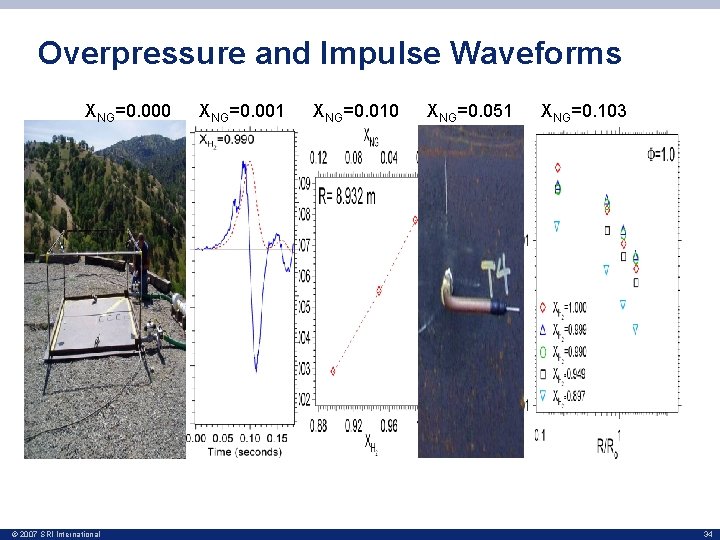
Overpressure and Impulse Waveforms XNG=0. 000 © 2007 SRI International XNG=0. 001 XNG=0. 010 XNG=0. 051 XNG=0. 103 34

Sachs Scaling Sachs scaled surface burst overpressure and impulse: Scaled Range E = energy release, J Po = ambient pressure, Pa The energy release was calculated based on the volume of the enclosure and the net heat of combustion of the fuel mixture © 2007 SRI International 35

Sachs Scaling Sachs Scaled Pressure Po = ambient pressure, Pa Sachs Scaled Impulse I (k. Pa-s) is impulse Co (m/s) is the speed of sound © 2007 SRI International 36

Sachs Scaled Overpressure and Impulse © 2007 SRI International 37

Scaled Overpressure © 2007 SRI International 38

Scaled Impulse © 2007 SRI International 39

Sachs Scaled Overpressure and Impulse • 0. 897 ≤ XH 2 ≤ 0. 949 –Hydrogen combustion was inhibited –Generated overpressures decreased significantly • 0. 990 ≤ XH 2 ≤ 1. 000 –Slight increase in overpressure –Increase was small –Could possibly be attributed to experimental scatter –Possible that there is a slight enhancement of the deflagration for these mixtures –Magnitude differences of the overpressures are small and should have little effect on safety considerations © 2007 SRI International 40

Summary • Fast response thermocouples used to measure flame front TOA – Velocities ranged from 19. 7 m/s to 36. 2 m/s – Thermocouple data is in good agreement with IR video data • The results show a decrease in flame speed with the addition of natural gas for fuel mixtures with XH 2 ≤ 0. 990 • The test with XH 2 = 0. 999 showed an increase in the flame speed with the addition of a small amount of natural gas © 2007 SRI International 41

Summary • For tests with XH 2 ≤ 0. 949 the overpressures decreased significantly with the substitution of natural gas for hydrogen • When the amount of natural gas substitution was small, 0. 990 ≤ XH 2 ≤ 0. 999, there was a slight increase in overpressure compared to the case with the stoichiometric hydrogen air mixture – Could be due to experimental scatter – Could be a slight enhancement when small amounts of natural gas are mixed with the hydrogen • More tests are necessary to determine whether the increased overpressure is due to scatter or enhancement • Regardless of the cause, the difference in overpressure was small and should not affect safety considerations © 2007 SRI International 42

Thank You Questions? © 2007 SRI International