Definitive treatments for SCD An era of multiple







- Slides: 27

‘Definitive treatments for SCD: An era of multiple choices’ __________ Sandeep Soni, MD Associate Prof. (Clin. ) of Pediatrics Div. of SCT and Regenerative Medicine Lucile Packard Children’s Hospital Stanford University, CA COI: Scientific Advisor Board –Crispr Tx, Inc. 1

Objectives of the Session 1. Provide an overview of definitive treatments with focus on alternative donor SCT and ex-vivo modification of HSPCs 2. Compare and contrast SCT and Gene-therapy 3. Update on Stanford initiatives 4. Risks-Benefits of different techniques 2

Sickle Cell Disease- Paradigm shift “Rather than an ‘episodic’ disease, SCD is a chronic inflammatory state leading to continuous organ damage, poor Qo. L and decreased lifeexpectancy’’ • Increased need for definitive options • Early intervention- prior to organ damage

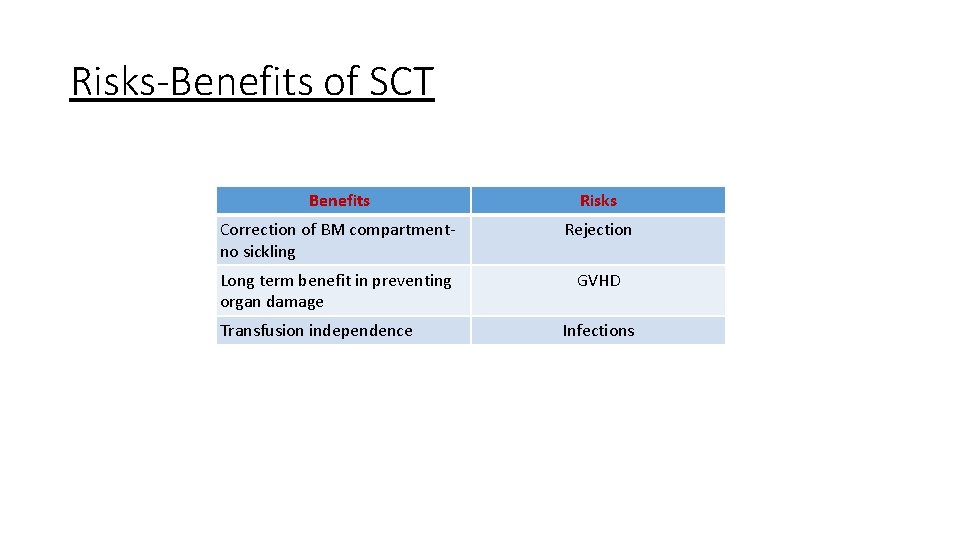
Risks-Benefits of SCT Benefits Risks Correction of BM compartmentno sickling Rejection Long term benefit in preventing organ damage GVHD Transfusion independence Infections

Allogeneic MSD SCT Overall survival: ~95%; DFS: ~90% Message: ‘With the current level of success, HSCT should be offered to all ‘severe’ SCD patients who have a matched sibling donor and an opportunity to save cord blood should be provided to all newly pregnant mothers who have a child with SCD’ Issue: MSD availability? Siblings with Hb. S trait can be donors also 5

Alternative Donor Transplants • Unrelated donor (NMDP registry) - Probability of finding HLA-match: ~33% • Unrelated cord blood (NMDP registry) - Low cell dose; risk of rejection • Haplo-identical SCT - Donor readily available

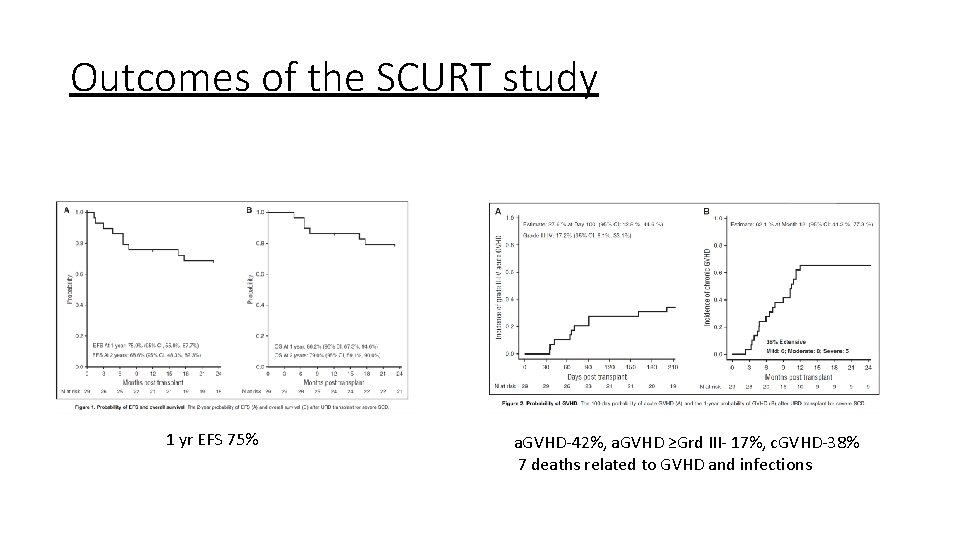
Outcomes of the SCURT study 1 yr EFS 75% a. GVHD-42%, a. GVHD ≥Grd III- 17%, c. GVHD-38% 7 deaths related to GVHD and infections

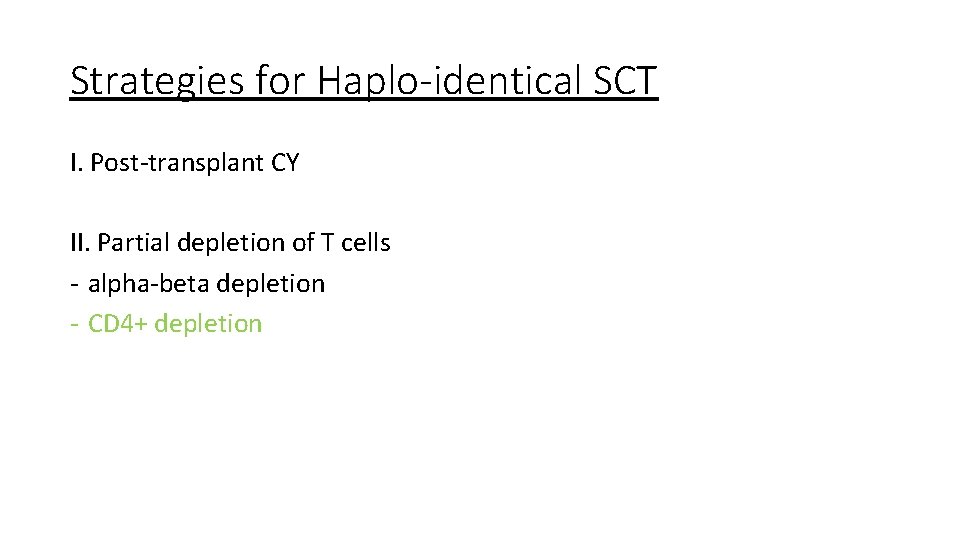
Strategies for Haplo-identical SCT I. Post-transplant CY II. Partial depletion of T cells - alpha-beta depletion - CD 4+ depletion

Results of alternative donor transplants • Rejection and GVHD still remain an issue Message: • Results of alternative donor transplants still need improvement • Should be performed on a study (clinical trial)

SCT options for SCD at Stanford Stem-Cell Source MRD HPC-C SOC: RIC 1 HPC-M SOC: RIC 1 UD Ex. Cellthera trial 2 x HPC-A x x Haplo-identical x x Alpha/Beta depletion trial 3 1 RIC: Campath+ Flu+ Mel ± Thiotepa 2 Ex. Cell. Thera: UM 171 expanded CD 34+ cells from unrelated cord blood using RIC regimen for SCD 3 Alpha/beta depletion trial for haploidentical transplant 10

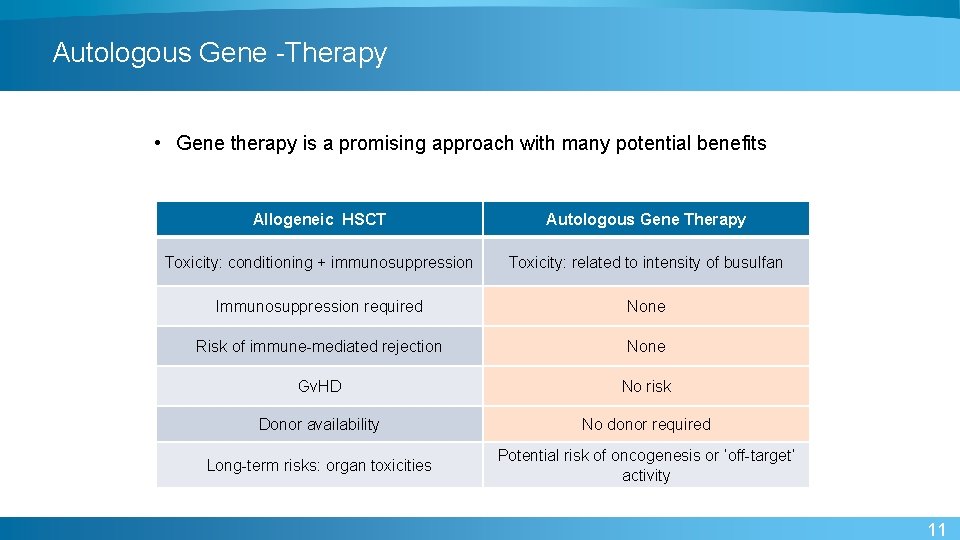
Autologous Gene -Therapy • Gene therapy is a promising approach with many potential benefits Allogeneic HSCT Autologous Gene Therapy Toxicity: conditioning + immunosuppression Toxicity: related to intensity of busulfan Immunosuppression required None Risk of immune-mediated rejection None Gv. HD No risk Donor availability No donor required Long-term risks: organ toxicities Potential risk of oncogenesis or ‘off-target’ activity 11

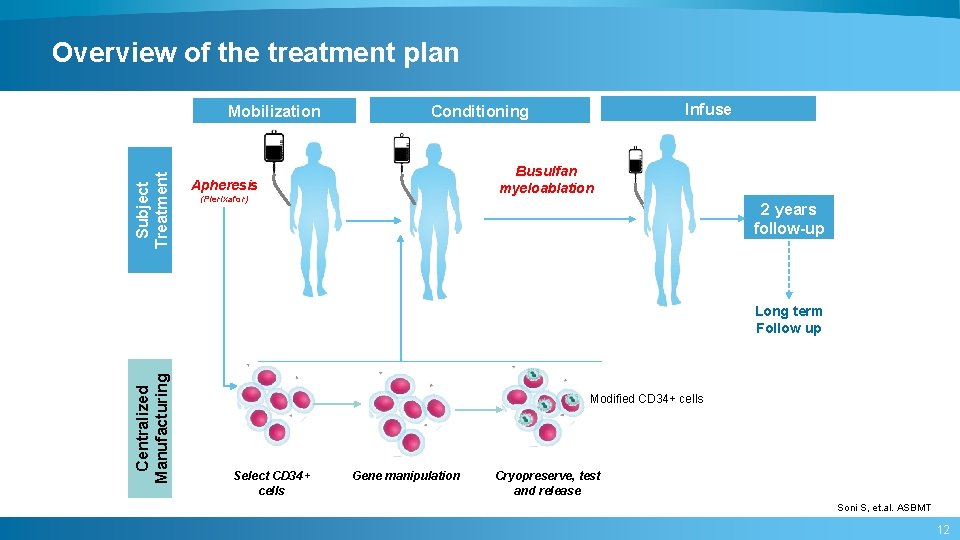
Overview of the treatment plan Subject Treatment Mobilization Infuse Conditioning Busulfan myeloablation Apheresis (Plerixafor) 2 years follow-up Centralized Manufacturing Long term Follow up Modified CD 34+ cells Select CD 34+ cells Gene manipulation Cryopreserve, test and release Soni S, et. al. ASBMT 12

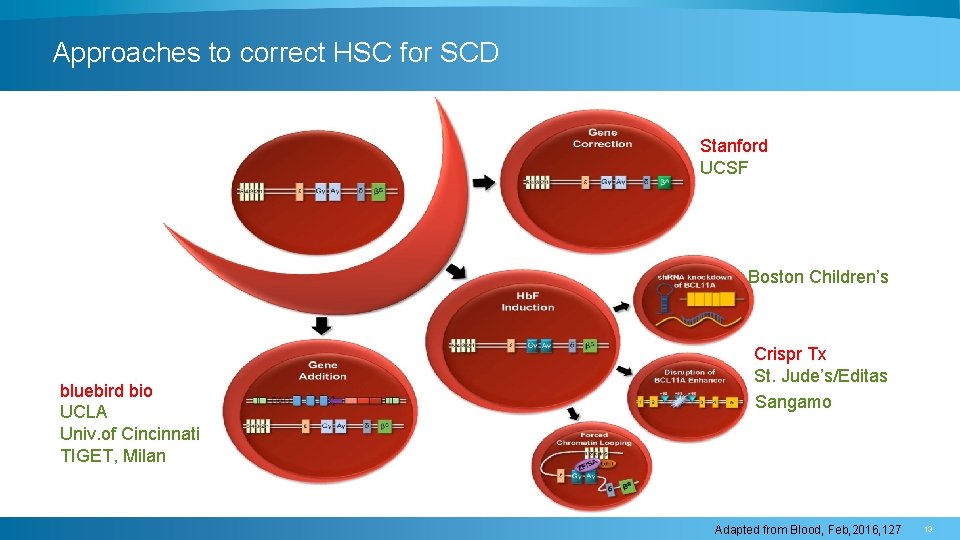
Approaches to correct HSC for SCD Stanford UCSF Boston Children’s bluebird bio UCLA Univ. of Cincinnati TIGET, Milan Crispr Tx St. Jude’s/Editas Sangamo Adapted from Blood, Feb, 2016, 127 13

Safety of the Lentivirus vectors for gene insertion ▪ Semi-random insertions: ‘ safe-sites’ for insertions? ▪ No Leukemia reported ~250 patients treated with lentivirus vector based GT in the last 5 years ▪ 1 patient in trial has developed MDS ▪ No RCL (HIV infection) till date ▪ FDA requires 15 years follow-up 14

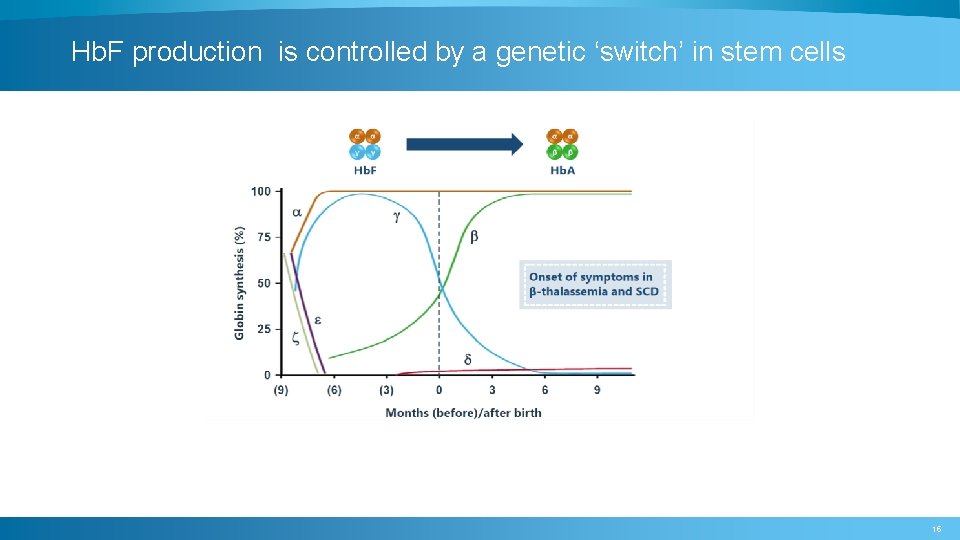
Hb. F production is controlled by a genetic ‘switch’ in stem cells 15

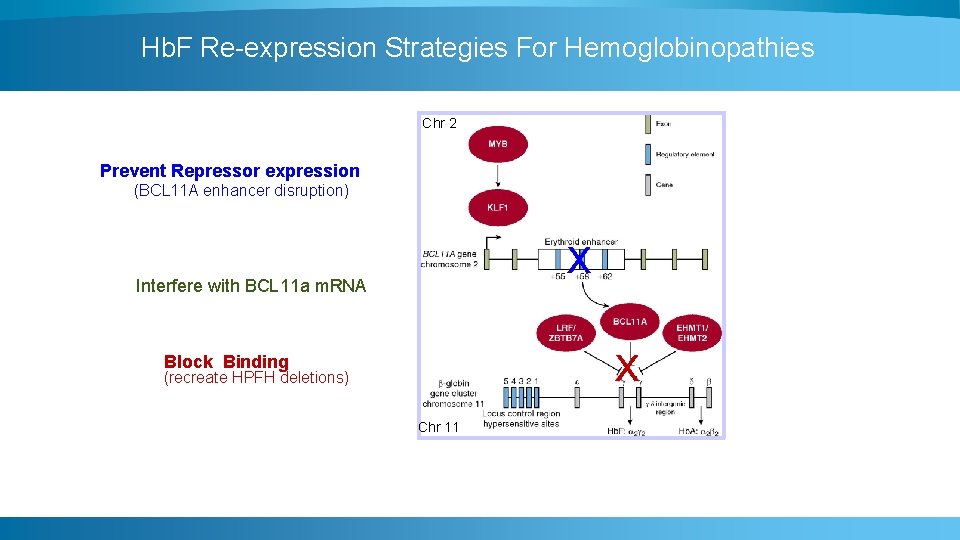
Hb. F Re-expression Strategies For Hemoglobinopathies Chr 2 Prevent Repressor expression (BCL 11 A enhancer disruption) x Interfere with BCL 11 a m. RNA x Block Binding (recreate HPFH deletions) Chr 11

BCH approach: RNAi technology Lentiviral vector to deliver sh. RNA segment to inhibit the BCL 11 a m. RNA Block BCL 11 a in erythroid progenitors; inhibitory-RNA tagged to HBB promoter (erythroid specific) Goal to increase Hb F production in red cells N=4 patients enrolled First patient: F-cells (>25% Hb. F): 80% in peripheral blood Resolution of symptoms BCL 11 a : a good target to increase Hb. F

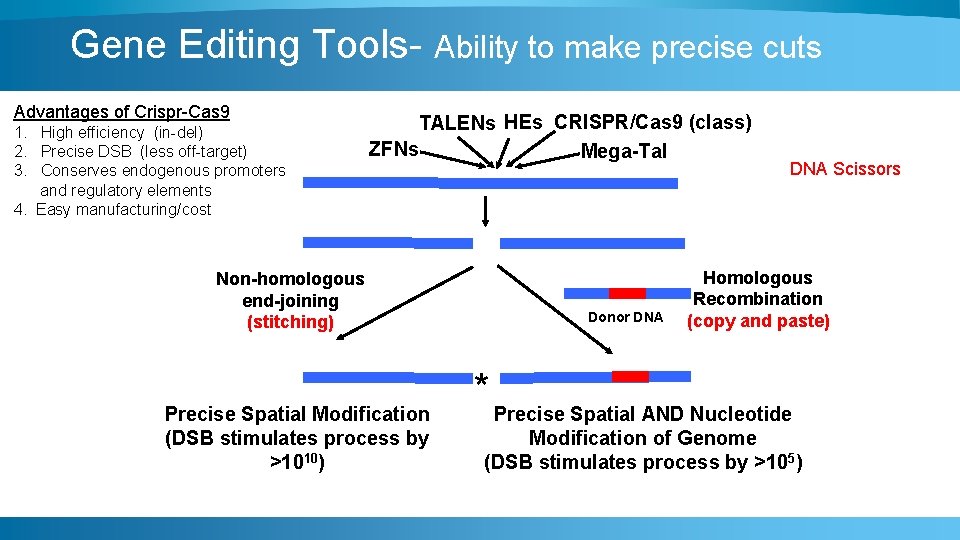
Gene Editing Tools- Ability to make precise cuts Advantages of Crispr-Cas 9 1. High efficiency (in-del) 2. Precise DSB (less off-target) 3. Conserves endogenous promoters and regulatory elements 4. Easy manufacturing/cost TALENs HEs CRISPR/Cas 9 (class) ZFNs Mega-Tal Non-homologous end-joining (stitching) Precise Spatial Modification (DSB stimulates process by >1010) Donor DNA Scissors Homologous Recombination (copy and paste) * Precise Spatial AND Nucleotide Modification of Genome (DSB stimulates process by >105)

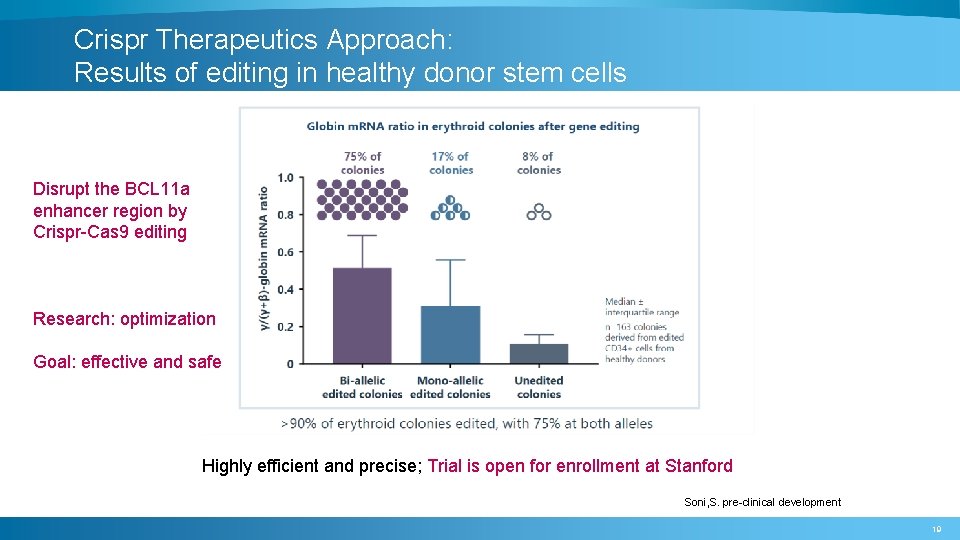
Crispr Therapeutics Approach: Results of editing in healthy donor stem cells Disrupt the BCL 11 a enhancer region by Crispr-Cas 9 editing Research: optimization Goal: effective and safe Highly efficient and precise; Trial is open for enrollment at Stanford Soni, S. pre-clinical development 19

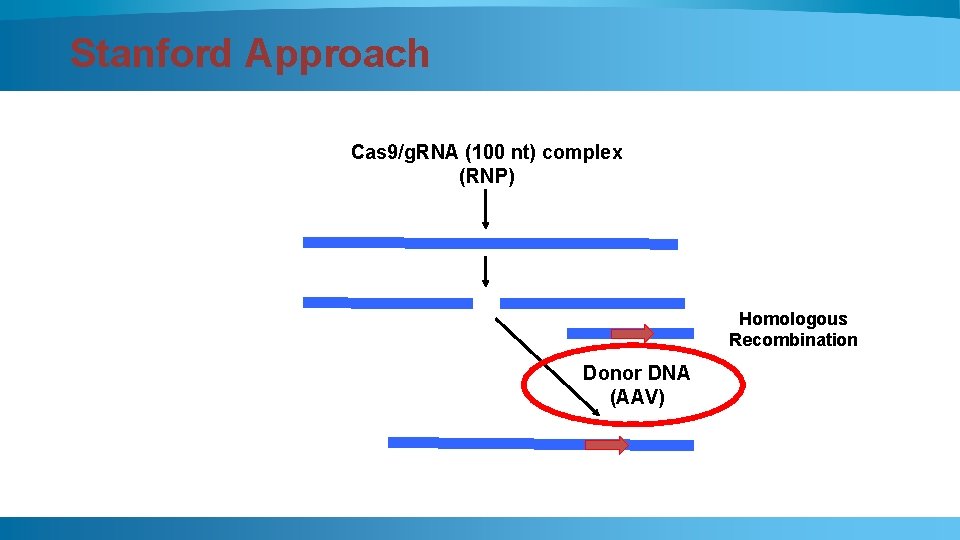
Stanford Approach Cas 9/g. RNA (100 nt) complex (RNP) Homologous Recombination Donor DNA (AAV)

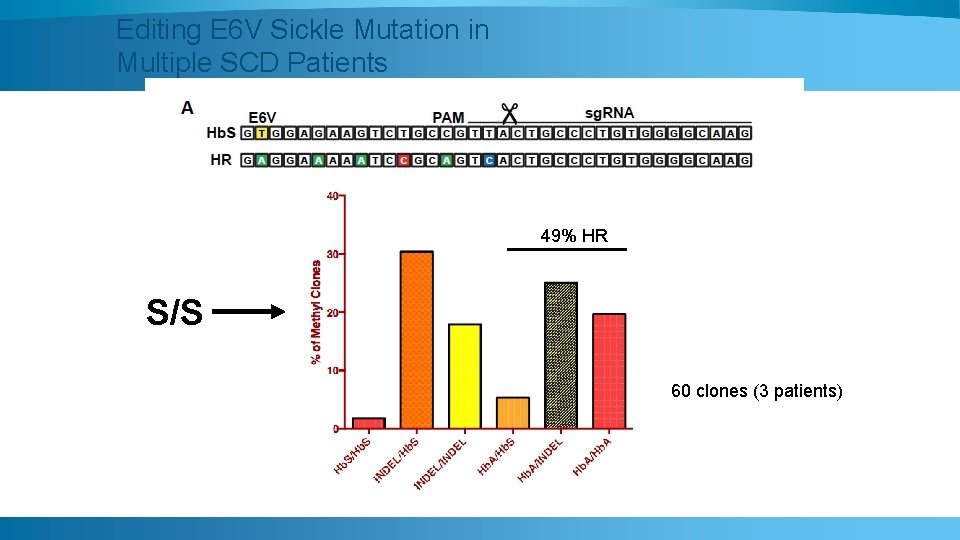
Editing E 6 V Sickle Mutation in Multiple SCD Patients 49% HR S/S 60 clones (3 patients)

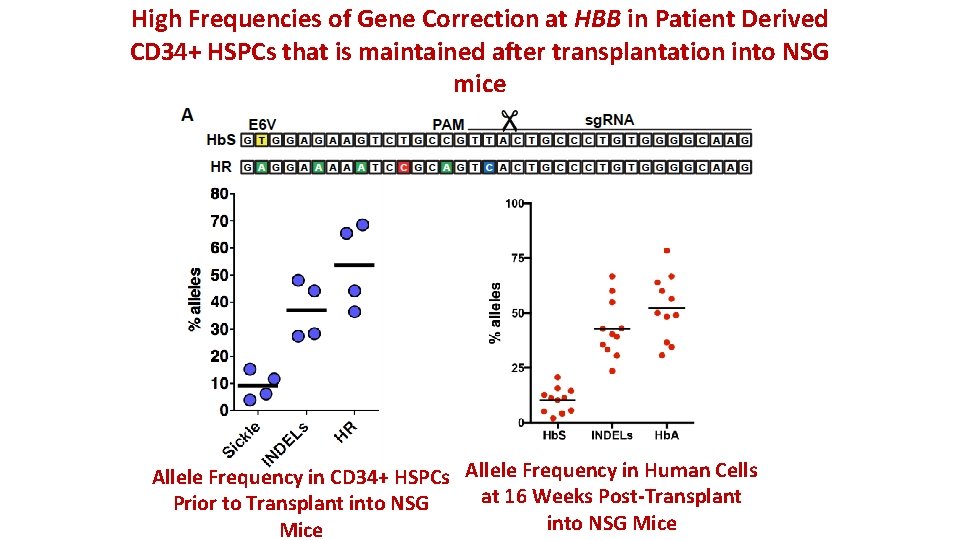
High Frequencies of Gene Correction at HBB in Patient Derived CD 34+ HSPCs that is maintained after transplantation into NSG mice Allele Frequency in CD 34+ HSPCs Allele Frequency in Human Cells at 16 Weeks Post-Transplant Prior to Transplant into NSG Mice

Key Takeaways • Multiple gene-insertion and editing approaches ‘targets and tools’ available • Long term benefit and safety are the key issues All clinical trials are fairly new • Crispr-Cas 9 approach: easy manufacturing, high efficiency and precision of editing, minimal toxicity in h. HSC 23

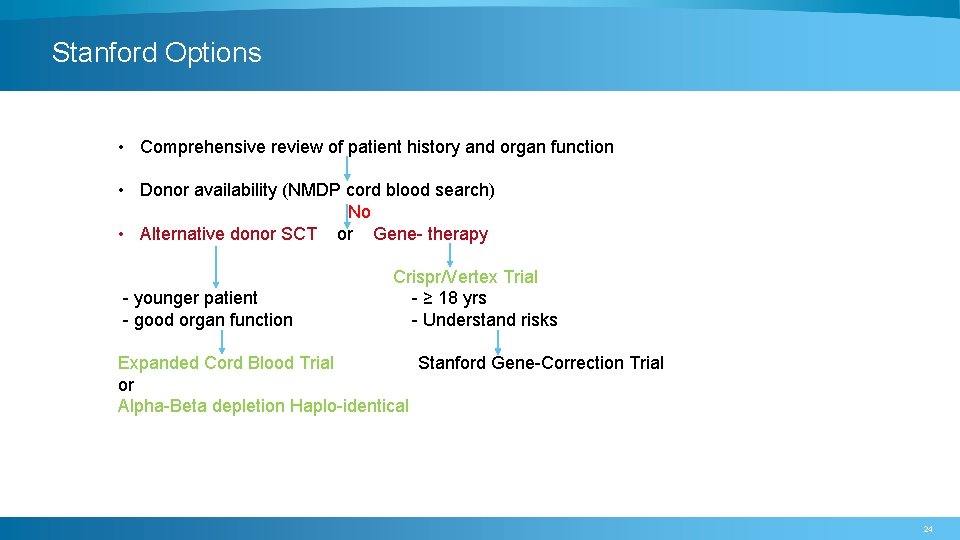
Stanford Options • Comprehensive review of patient history and organ function • Donor availability (NMDP cord blood search) No • Alternative donor SCT or Gene- therapy - younger patient - good organ function Crispr/Vertex Trial - ≥ 18 yrs - Understand risks Expanded Cord Blood Trial Stanford Gene-Correction Trial or Alpha-Beta depletion Haplo-identical 24

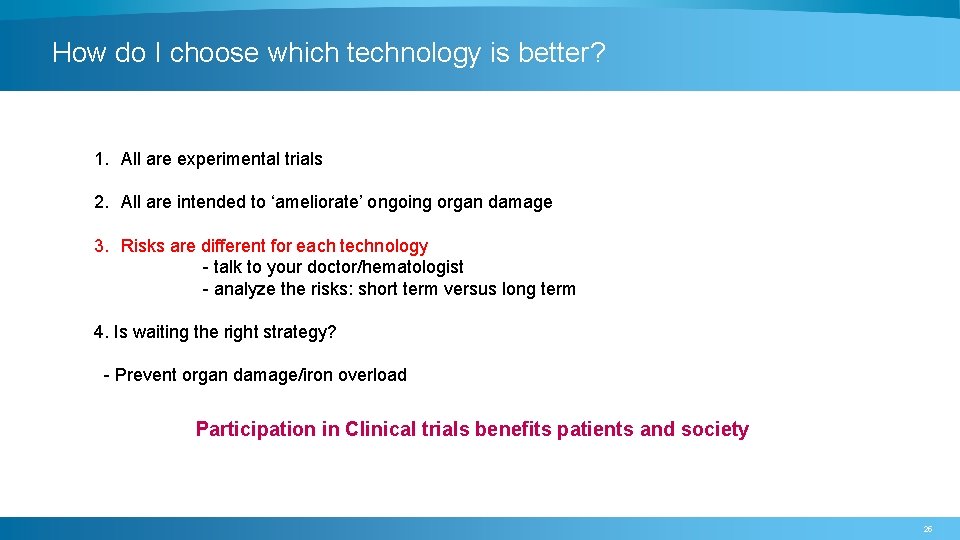
How do I choose which technology is better? 1. All are experimental trials 2. All are intended to ‘ameliorate’ ongoing organ damage 3. Risks are different for each technology - talk to your doctor/hematologist - analyze the risks: short term versus long term 4. Is waiting the right strategy? - Prevent organ damage/iron overload Participation in Clinical trials benefits patients and society 25

Questions

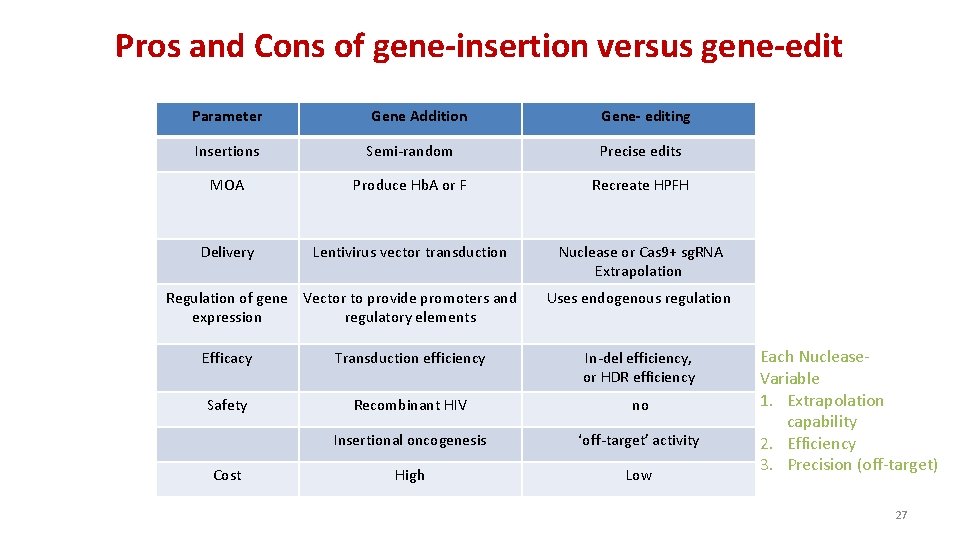
Pros and Cons of gene-insertion versus gene-edit Parameter Gene Addition Gene- editing Insertions Semi-random Precise edits MOA Produce Hb. A or F Recreate HPFH Delivery Lentivirus vector transduction Nuclease or Cas 9+ sg. RNA Extrapolation Regulation of gene Vector to provide promoters and expression regulatory elements Uses endogenous regulation Efficacy Transduction efficiency In-del efficiency, or HDR efficiency Safety Recombinant HIV no Insertional oncogenesis ‘off-target’ activity High Low Cost Each Nuclease. Variable 1. Extrapolation capability 2. Efficiency 3. Precision (off-target) 27