Definitions Solution homogeneous mixture Solute substance being dissolved
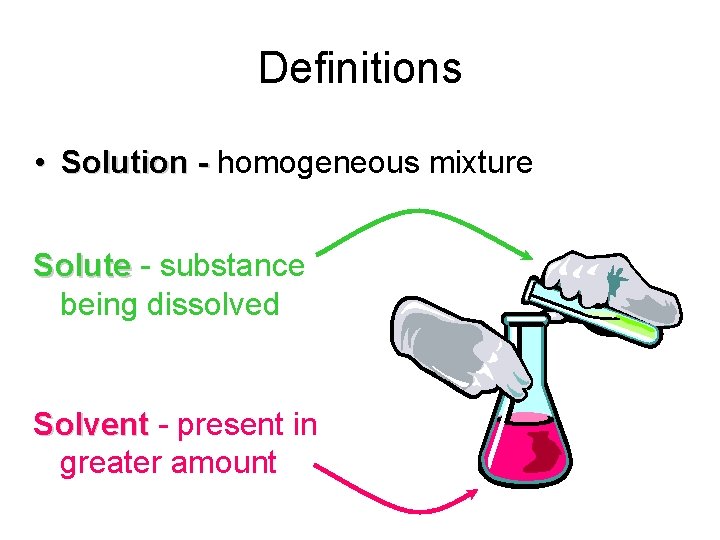
Definitions • Solution - homogeneous mixture Solute - substance being dissolved Solvent - present in greater amount

Solution = Solute + Solvent • Solute - gets dissolved • Solvent - does the dissolving – Aqueous – Tincture – Amalgam – Organic • Polar • Non-polar (water) (alcohol) (mercury) Dental filling
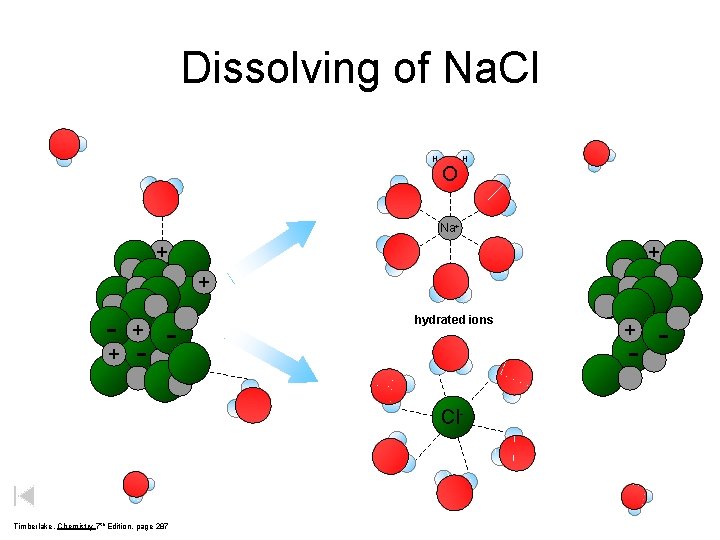
Dissolving of Na. Cl H O H Na+ + - - hydrated ions - Cl- Timberlake, Chemistry 7 th Edition, page 287 + -
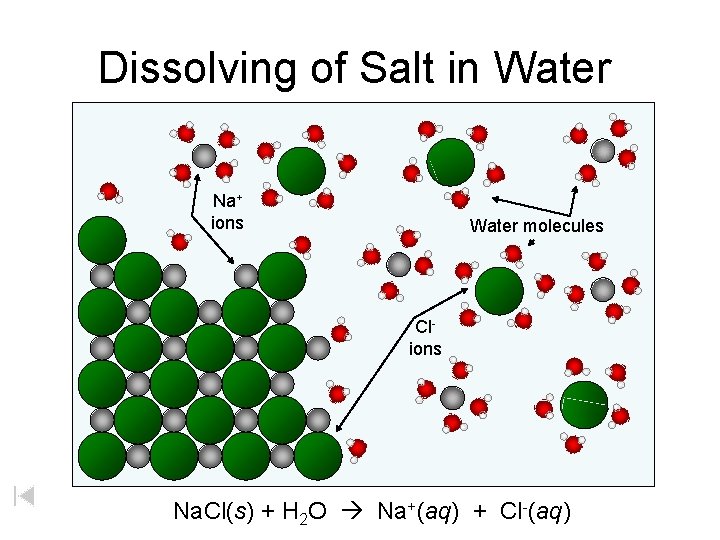
Dissolving of Salt in Water Na+ ions Water molecules Clions Na. Cl(s) + H 2 O Na+(aq) + Cl-(aq)
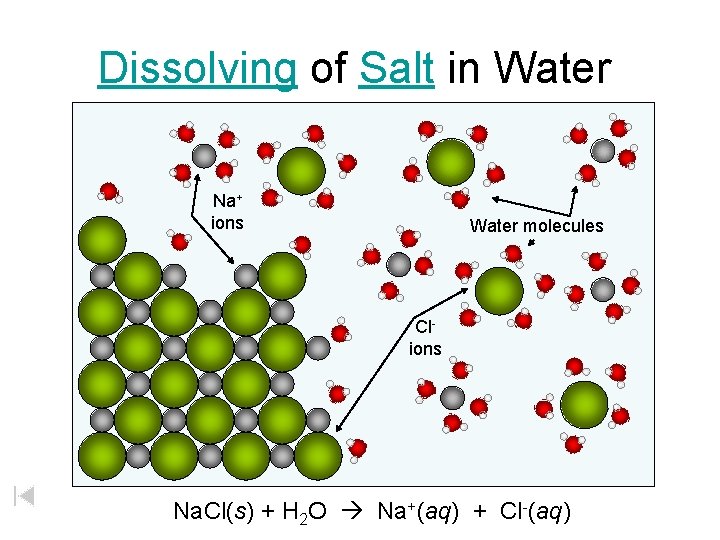
Dissolving of Salt in Water Na+ ions Water molecules Clions Na. Cl(s) + H 2 O Na+(aq) + Cl-(aq)
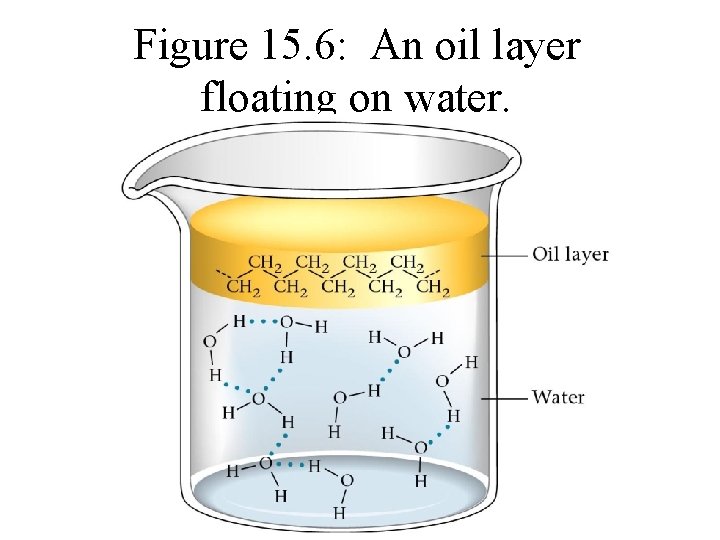
Figure 15. 6: An oil layer floating on water.
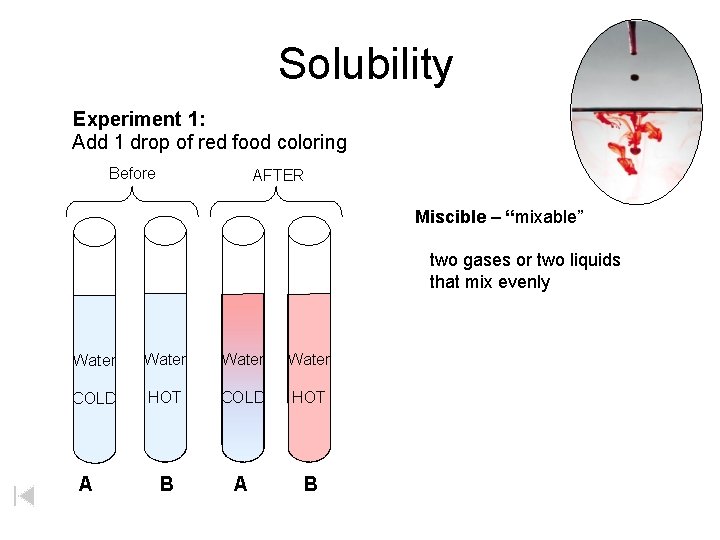
Solubility Experiment 1: Add 1 drop of red food coloring Before AFTER Miscible – “mixable” two gases or two liquids that mix evenly Water COLD HOT B A
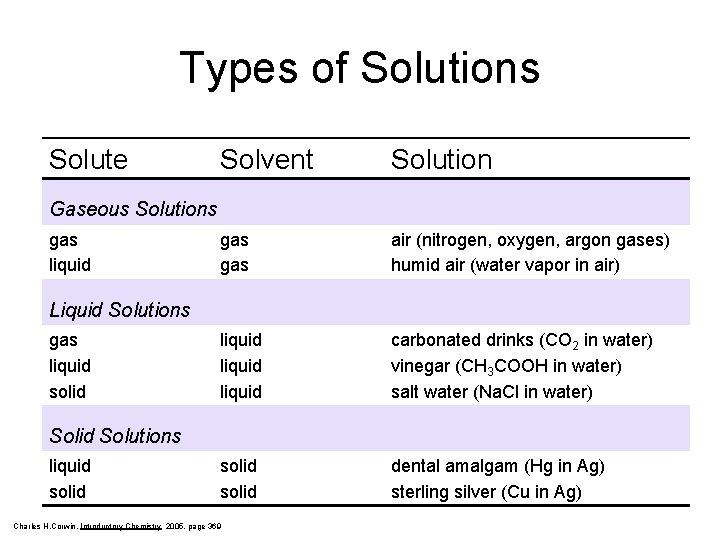
Types of Solutions Solute Solvent Solution gas air (nitrogen, oxygen, argon gases) humid air (water vapor in air) liquid carbonated drinks (CO 2 in water) vinegar (CH 3 COOH in water) salt water (Na. Cl in water) solid dental amalgam (Hg in Ag) sterling silver (Cu in Ag) Gaseous Solutions gas liquid Liquid Solutions gas liquid solid Solutions liquid solid Charles H. Corwin, Introductory Chemistry 2005, page 369
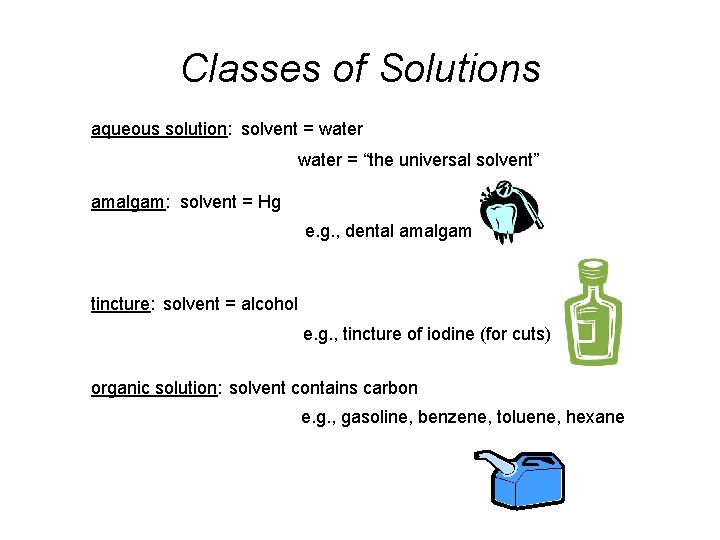
Classes of Solutions aqueous solution: solvent = water = “the universal solvent” amalgam: solvent = Hg e. g. , dental amalgam tincture: solvent = alcohol e. g. , tincture of iodine (for cuts) organic solution: solvent contains carbon e. g. , gasoline, benzene, toluene, hexane
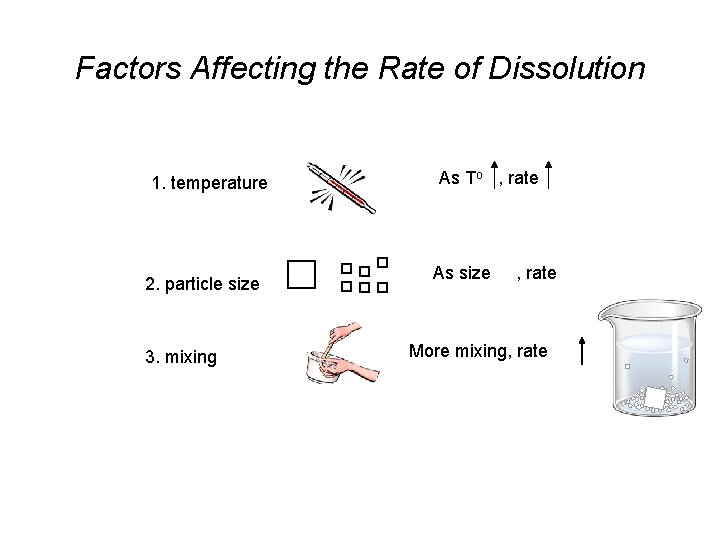
Factors Affecting the Rate of Dissolution 1. temperature 2. particle size 3. mixing As To , rate As size , rate More mixing, rate
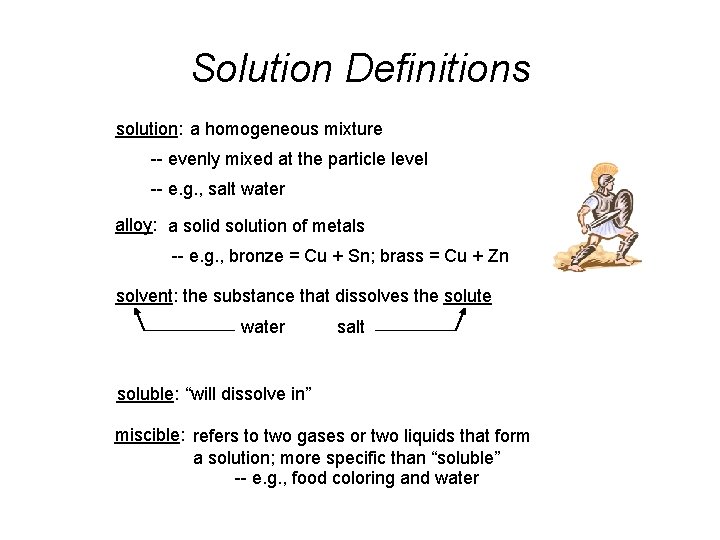
Solution Definitions solution: a homogeneous mixture -- evenly mixed at the particle level -- e. g. , salt water alloy: a solid solution of metals -- e. g. , bronze = Cu + Sn; brass = Cu + Zn solvent: the substance that dissolves the solute water salt soluble: “will dissolve in” miscible: refers to two gases or two liquids that form a solution; more specific than “soluble” -- e. g. , food coloring and water

Non-Solution Definitions insoluble: “will NOT dissolve in” e. g. , sand water immiscible: refers to two gases or two liquids that will NOT form a solution e. g. , water and oil suspension: appears uniform while being stirred, but settles over time
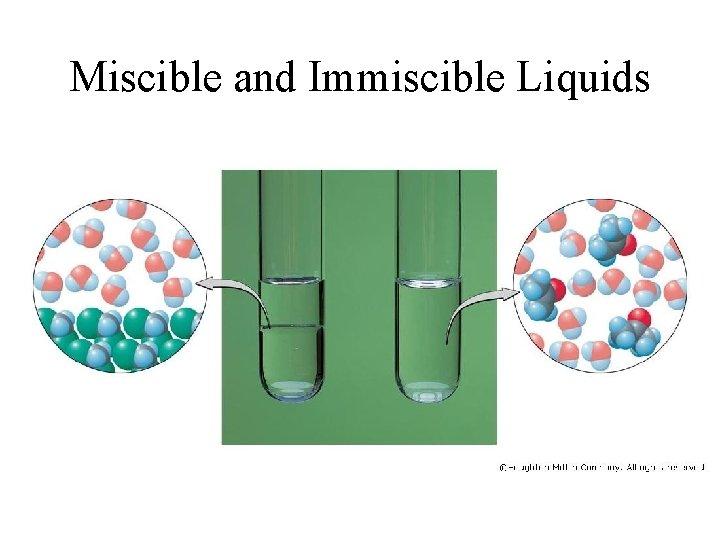
Miscible and Immiscible Liquids
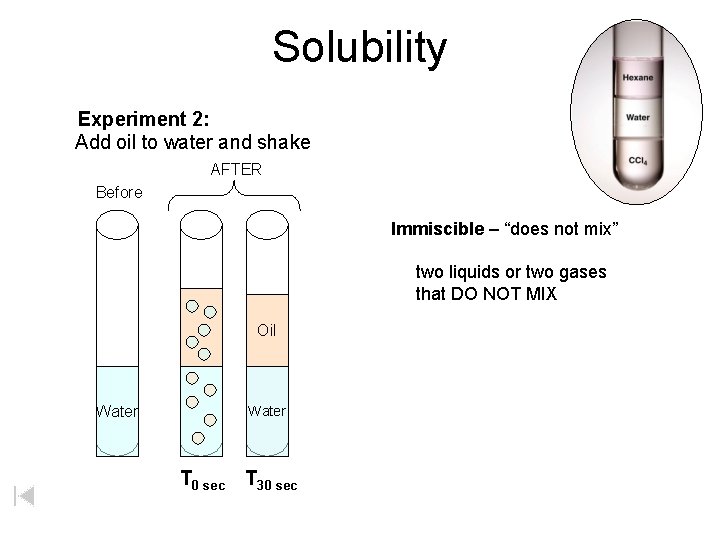
Solubility Experiment 2: Add oil to water and shake AFTER Before Immiscible – “does not mix” two liquids or two gases that DO NOT MIX Oil Water T 0 sec T 30 sec
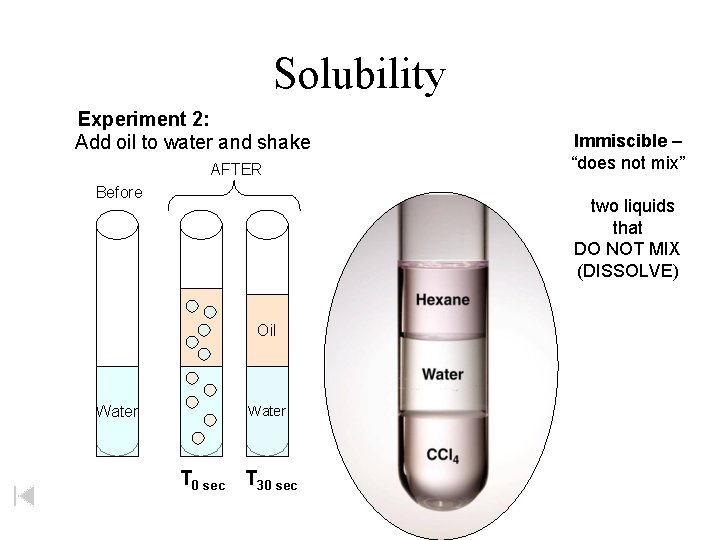
Solubility Experiment 2: Add oil to water and shake AFTER Before Immiscible – “does not mix” two liquids that DO NOT MIX (DISSOLVE) Oil Water T 0 sec T 30 sec
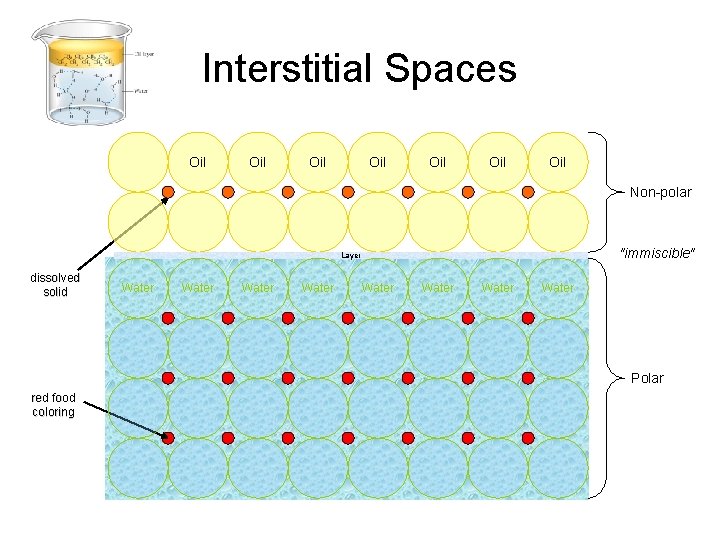
Interstitial Spaces Oil Oil Non-polar "immiscible" Layer dissolved solid Water Water Polar red food coloring
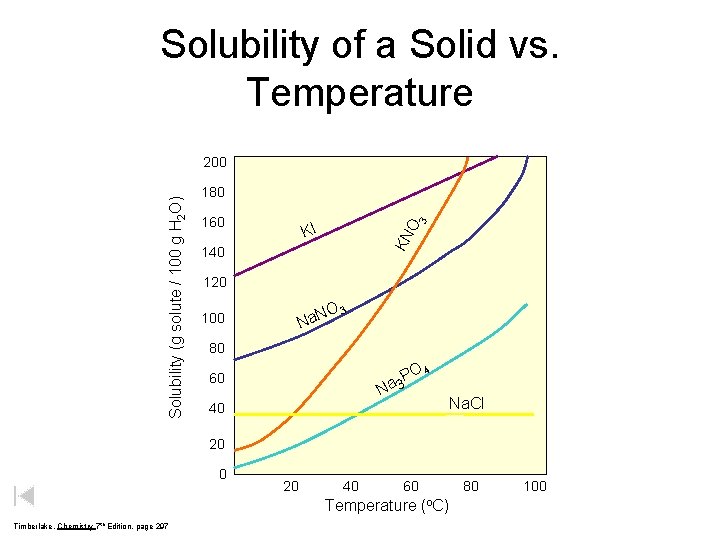
Solubility of a Solid vs. Temperature 180 160 O 3 KI KN Solubility (g solute / 100 g H 2 O) 200 140 120 O Na. N 100 3 80 O 4 P a 3 60 N Na. Cl 40 20 40 60 Temperature (o. C) Timberlake, Chemistry 7 th Edition, page 297 80 100
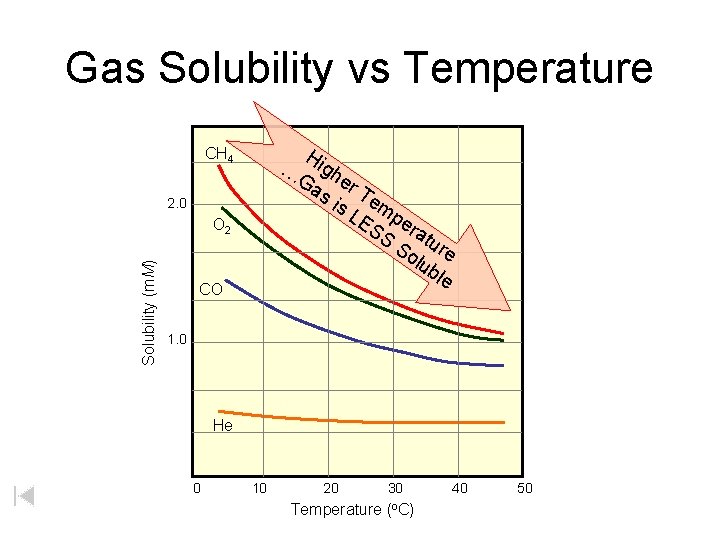
Gas Solubility vs Temperature Hi gh Ga er s i Te s L mp ES er S atu So re lub le CH 4 … 2. 0 Solubility (m. M) O 2 CO 1. 0 He 0 10 20 30 Temperature (o. C) 40 50
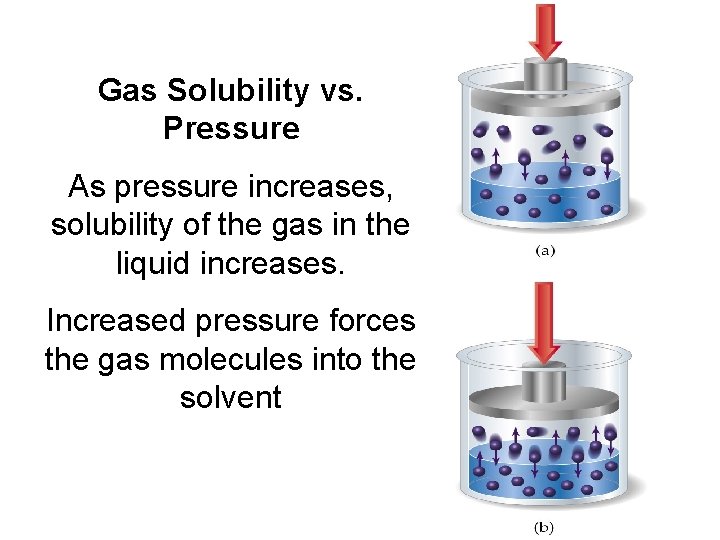
Gas Solubility vs. Pressure As pressure increases, solubility of the gas in the liquid increases. Increased pressure forces the gas molecules into the solvent

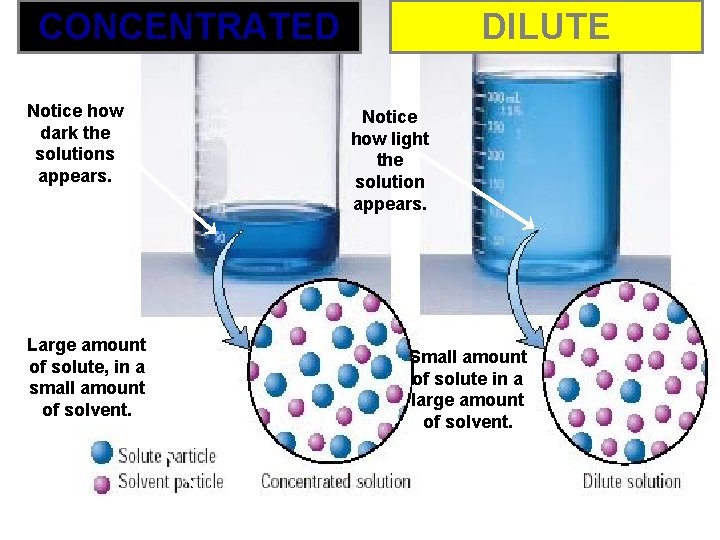
CONCENTRATED DILUTE Concentrated vs. Dilute Notice how dark the solutions appears. Large amount of solute, in a small amount of solvent. Notice how light the solution appears. Small amount of solute in a large amount of solvent.
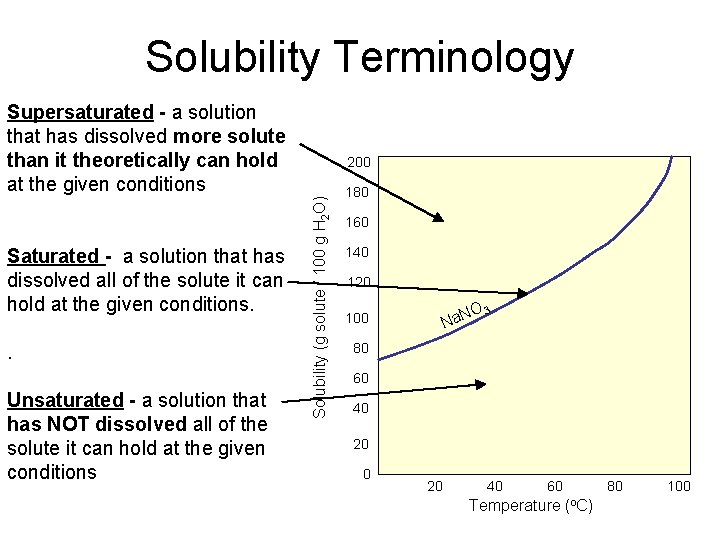
Solubility Terminology Supersaturated - a solution that has dissolved more solute than it theoretically can hold at the given conditions . Unsaturated - a solution that has NOT dissolved all of the solute it can hold at the given conditions Solubility (g solute / 100 g H 2 O) Saturated - a solution that has dissolved all of the solute it can hold at the given conditions. 200 180 160 140 120 O Na. N 100 3 80 60 40 20 40 60 Temperature (o. C) 80 100
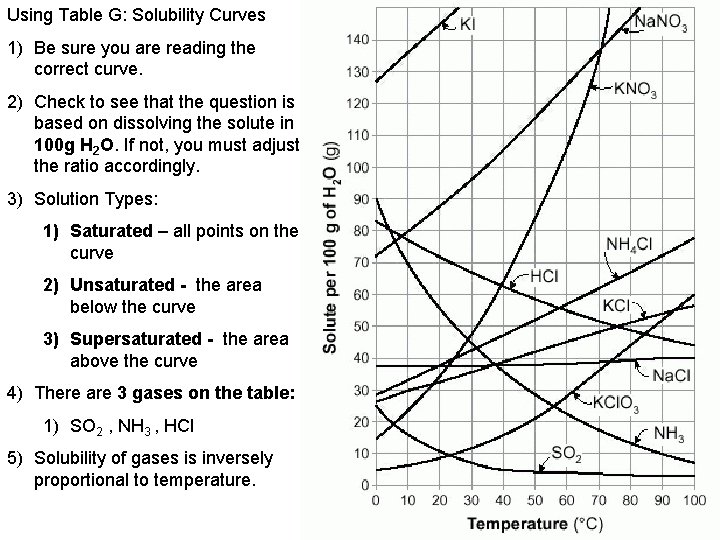
Using Table G: Solubility Curves 1) Be sure you are reading the correct curve. 2) Check to see that the question is based on dissolving the solute in 100 g H 2 O. If not, you must adjust the ratio accordingly. 3) Solution Types: 1) Saturated – all points on the curve 2) Unsaturated - the area below the curve 3) Supersaturated - the area above the curve 4) There are 3 gases on the table: 1) SO 2 , NH 3 , HCl 5) Solubility of gases is inversely proportional to temperature.
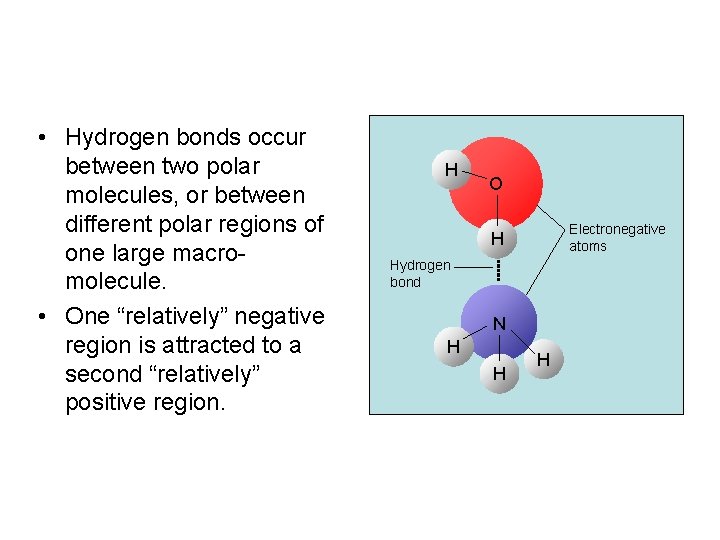
• Hydrogen bonds occur between two polar molecules, or between different polar regions of one large macromolecule. • One “relatively” negative region is attracted to a second “relatively” positive region. H O Electronegative atoms H Hydrogen bond N H H H
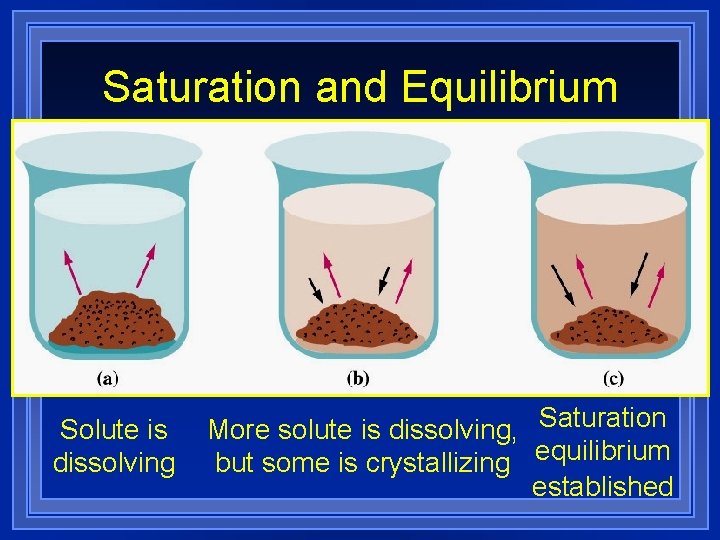
Saturation and Equilibrium Solute is dissolving More solute is dissolving, Saturation but some is crystallizing equilibrium established

35) 6 Quantitative Methods to Express Concentration 1) 2) 3) 4) 5) 6) Percent by Mass Percent by Volume Molarity (M) Molality (m) Mole Fraction Parts per Million (ppm)
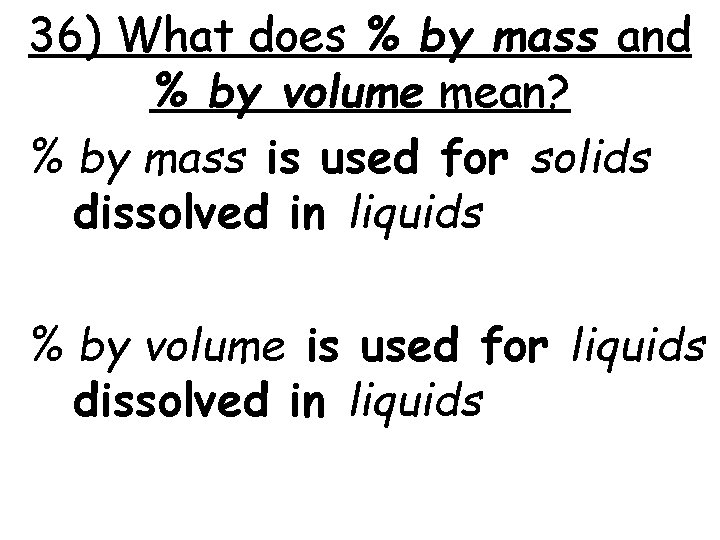
36) What does % by mass and % by volume mean? % by mass is used for solids dissolved in liquids % by volume is used for liquids dissolved in liquids

38) Define Molarity? Moles of solute per liter of solution. (Note: not per liter of solvent).
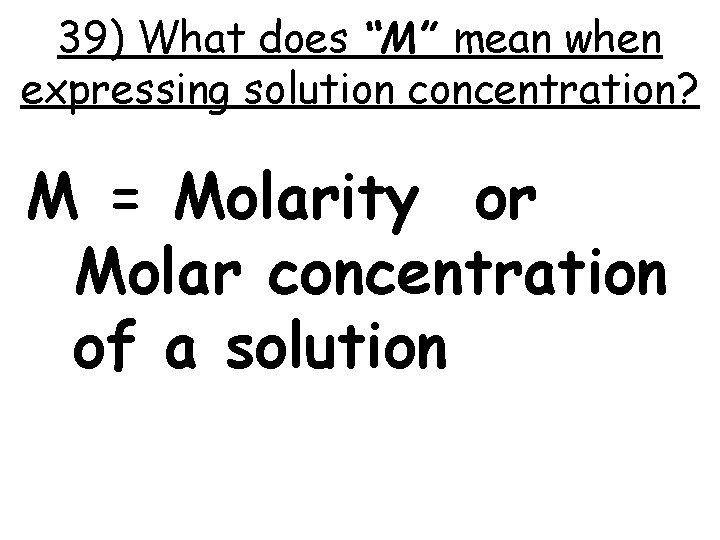
39) What does “M” mean when expressing solution concentration? M = Molarity or Molar concentration of a solution

40)Solving Molarity Problems a) Identify the given b) Change grams to moles c) Change m. L to liters d) Divide common units e) Give answer – pay attention to units!

41) Steps to follow a) convert grams to moles b) calculate molarity Molarity = Moles of Solute liter of solution

42) Steps to follow a) calculate number of moles needed Molarity = # of Moles liter of solution b) convert moles to grams
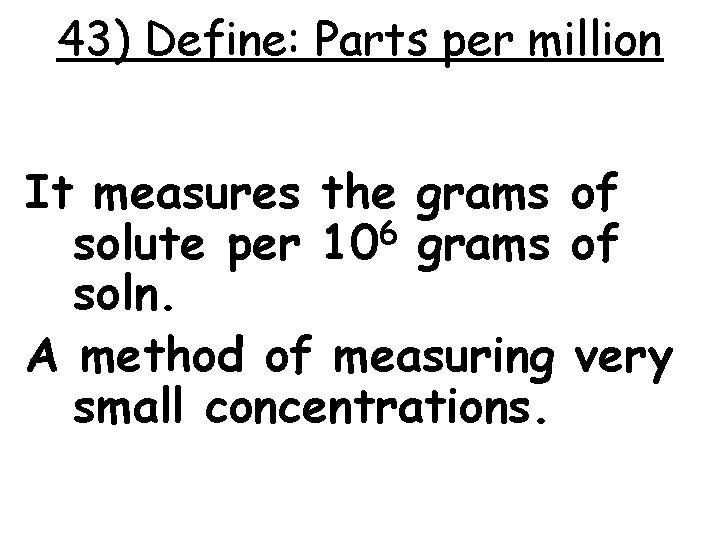
43) Define: Parts per million It measures the grams of solute per 106 grams of soln. A method of measuring very small concentrations.
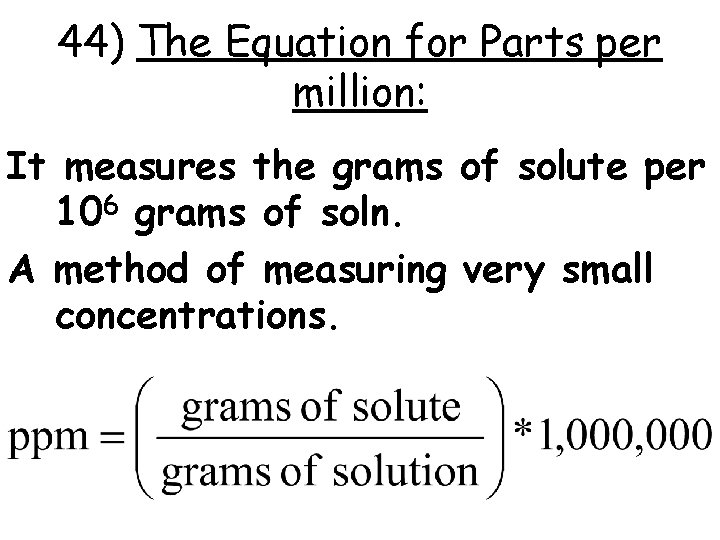
44) The Equation for Parts per million: It measures the grams of solute per 106 grams of soln. A method of measuring very small concentrations.

Some particles in solution will IONIZE (or split), while others may not. - Page 488 Colligative Properties Ca. Cl 2 will have Glucose will only have Na. Cl will have two three particles in solution for each one particle in particles in solution for each one particle it starts with.
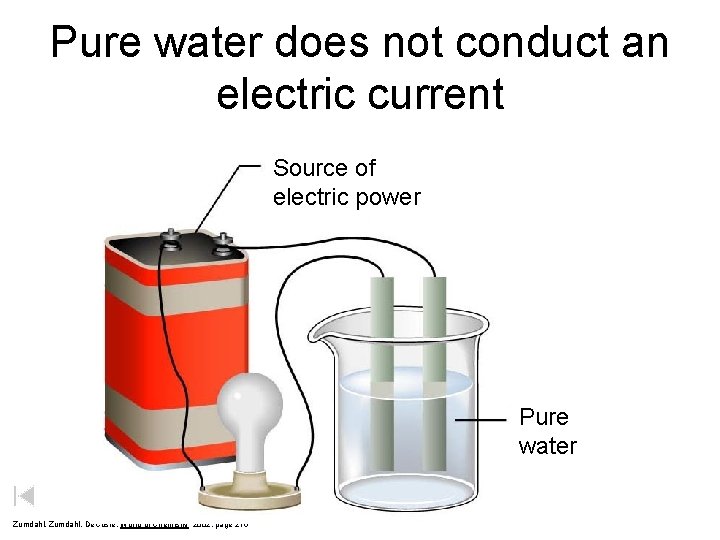
Pure water does not conduct an electric current Source of electric power Pure water Zumdahl, De. Coste, World of Chemistry 2002, page 215
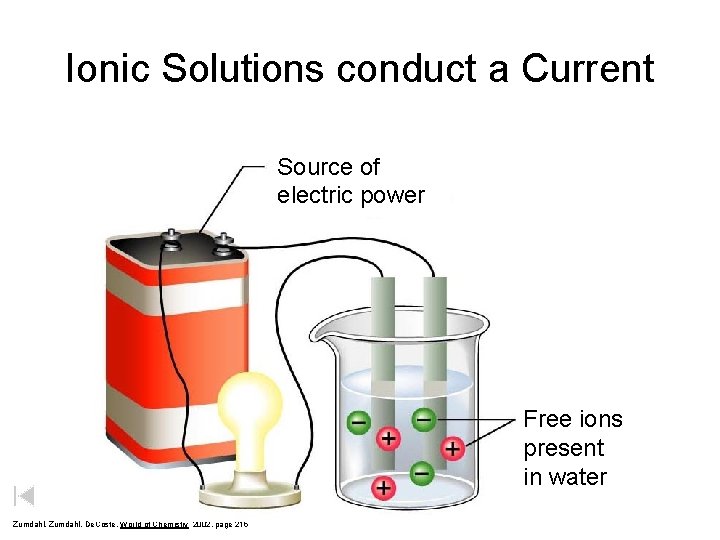
Ionic Solutions conduct a Current Source of electric power Free ions present in water Zumdahl, De. Coste, World of Chemistry 2002, page 215
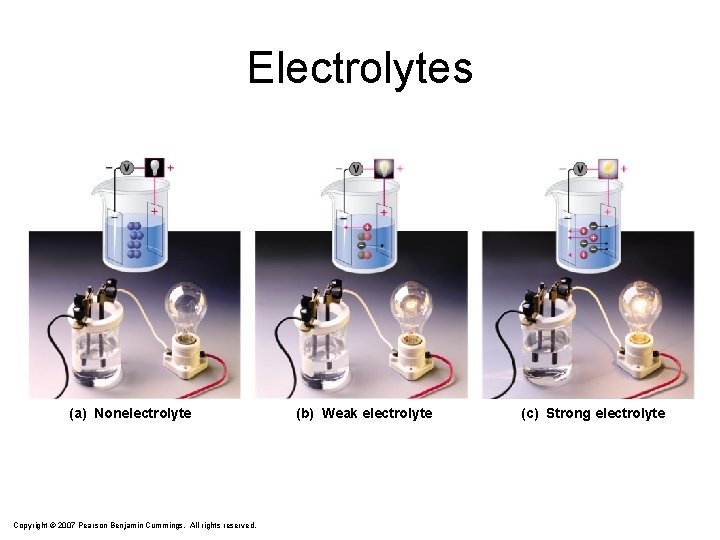
Electrolytes (a) Nonelectrolyte Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. (b) Weak electrolyte (c) Strong electrolyte
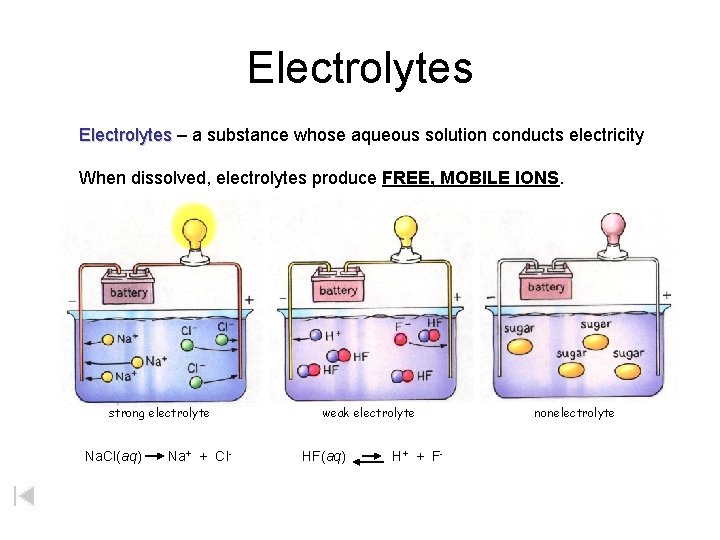
Electrolytes – a substance whose aqueous solution conducts electricity When dissolved, electrolytes produce FREE, MOBILE IONS. strong electrolyte Na. Cl(aq) Na+ + Cl- weak electrolyte HF(aq) H + + F- nonelectrolyte
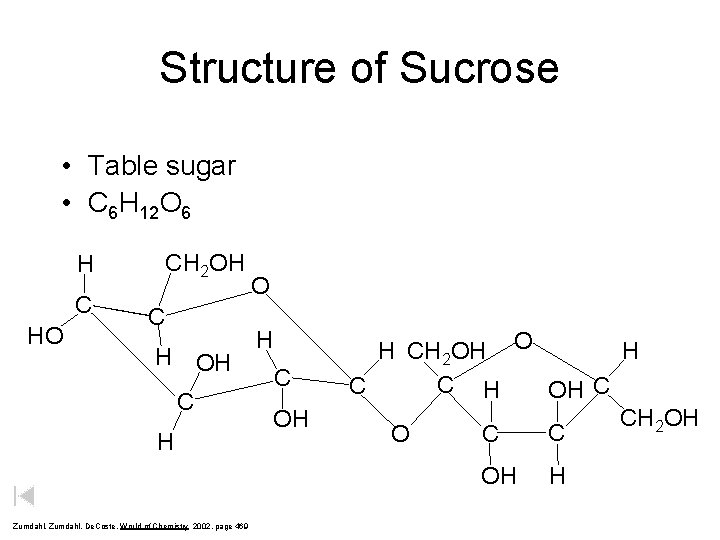
Structure of Sucrose • Table sugar • C 6 H 12 O 6 H C HO CH 2 OH C H O H C OH H H CH 2 OH O C H C OH C CH 2 OH C C O OH Zumdahl, De. Coste, World of Chemistry 2002, page 469 H
- Slides: 40