Definitions Mole amount of substance defined by the

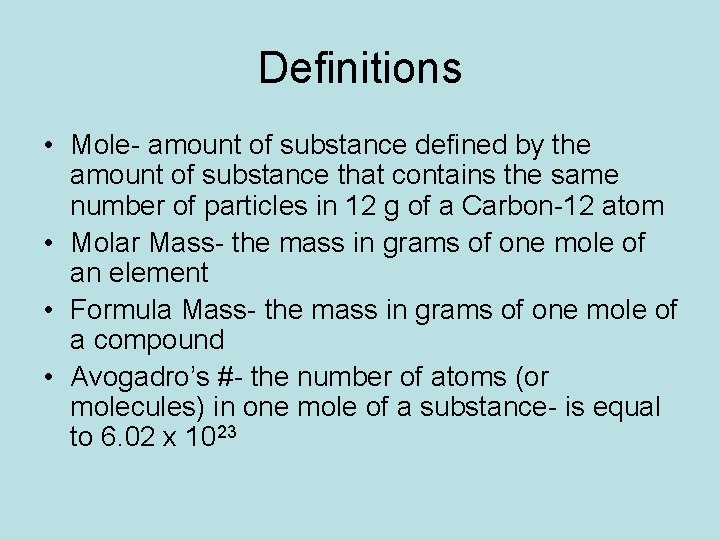
Definitions • Mole- amount of substance defined by the amount of substance that contains the same number of particles in 12 g of a Carbon-12 atom • Molar Mass- the mass in grams of one mole of an element • Formula Mass- the mass in grams of one mole of a compound • Avogadro’s #- the number of atoms (or molecules) in one mole of a substance- is equal to 6. 02 x 1023

Formula Mass Problems 1. 2. 3. 4. 5. 6. 7. 8. 68. 143 159. 6882 138. 5486 145. 9964 132. 1406 97. 995181 133. 339638 226. 26876 9. 98. 07948 10. 108. 01048 11. 249. 686 12. 154. 757 13. 186. 73936 14. 149. 086861 15. 232. 97886
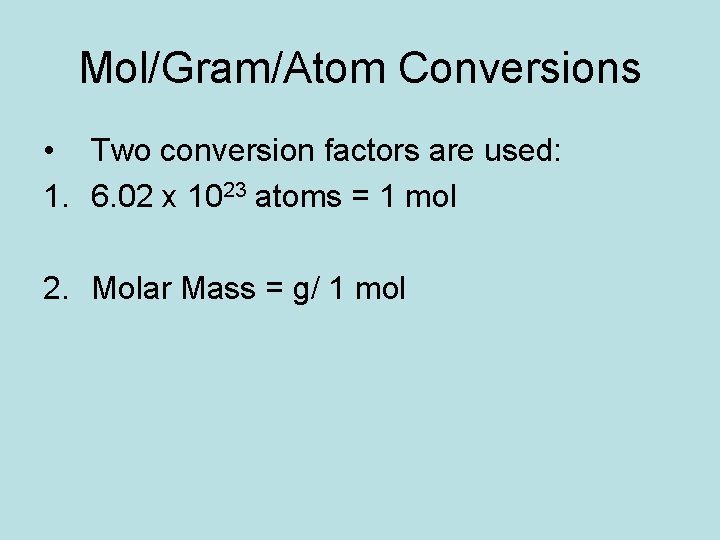
Mol/Gram/Atom Conversions • Two conversion factors are used: 1. 6. 02 x 1023 atoms = 1 mol 2. Molar Mass = g/ 1 mol
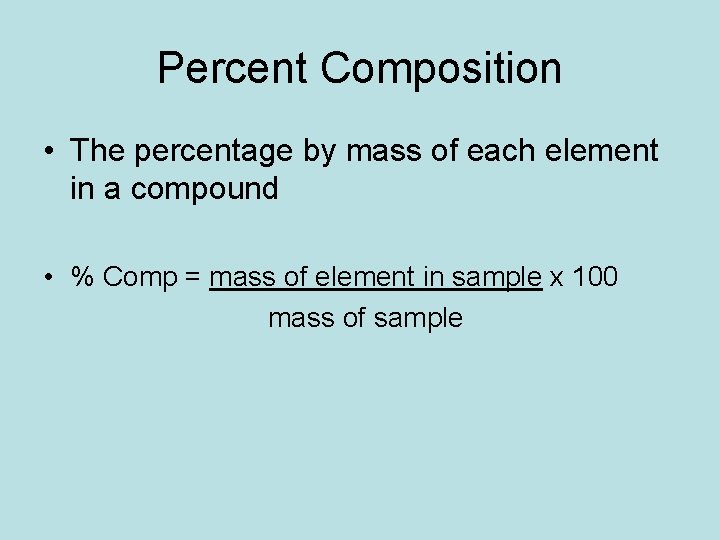
Percent Composition • The percentage by mass of each element in a compound • % Comp = mass of element in sample x 100 mass of sample
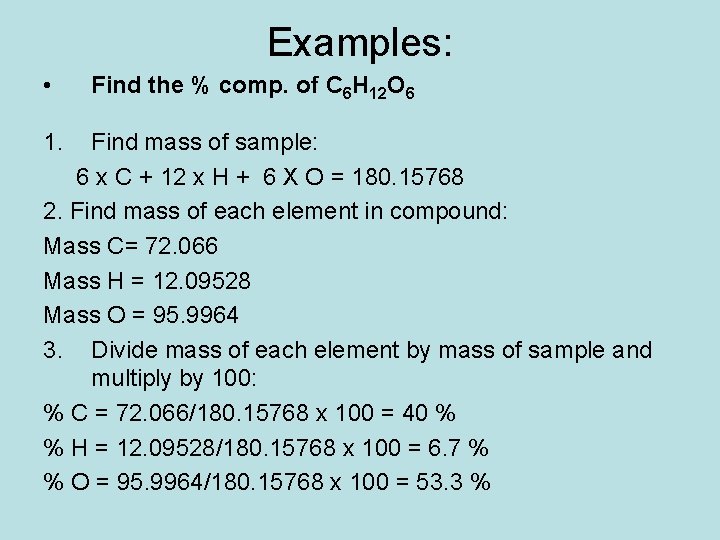
Examples: • 1. Find the % comp. of C 6 H 12 O 6 Find mass of sample: 6 x C + 12 x H + 6 X O = 180. 15768 2. Find mass of each element in compound: Mass C= 72. 066 Mass H = 12. 09528 Mass O = 95. 9964 3. Divide mass of each element by mass of sample and multiply by 100: % C = 72. 066/180. 15768 x 100 = 40 % % H = 12. 09528/180. 15768 x 100 = 6. 7 % % O = 95. 9964/180. 15768 x 100 = 53. 3 %

More Examples: • Find the % comp. of water in: Cu. SO 4. 5 H 2 O Mass of sample = 249. 686 g Mass of water = 90. 0764 g % comp. of water = 36 %
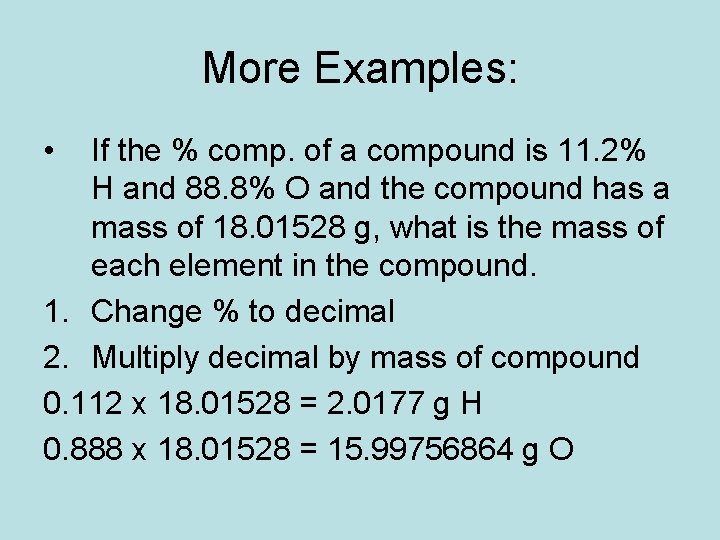
More Examples: • If the % comp. of a compound is 11. 2% H and 88. 8% O and the compound has a mass of 18. 01528 g, what is the mass of each element in the compound. 1. Change % to decimal 2. Multiply decimal by mass of compound 0. 112 x 18. 01528 = 2. 0177 g H 0. 888 x 18. 01528 = 15. 99756864 g O
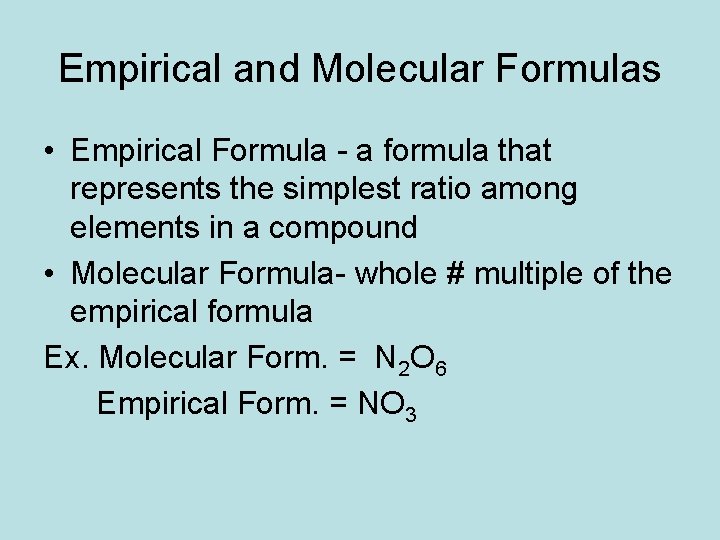
Empirical and Molecular Formulas • Empirical Formula - a formula that represents the simplest ratio among elements in a compound • Molecular Formula- whole # multiple of the empirical formula Ex. Molecular Form. = N 2 O 6 Empirical Form. = NO 3
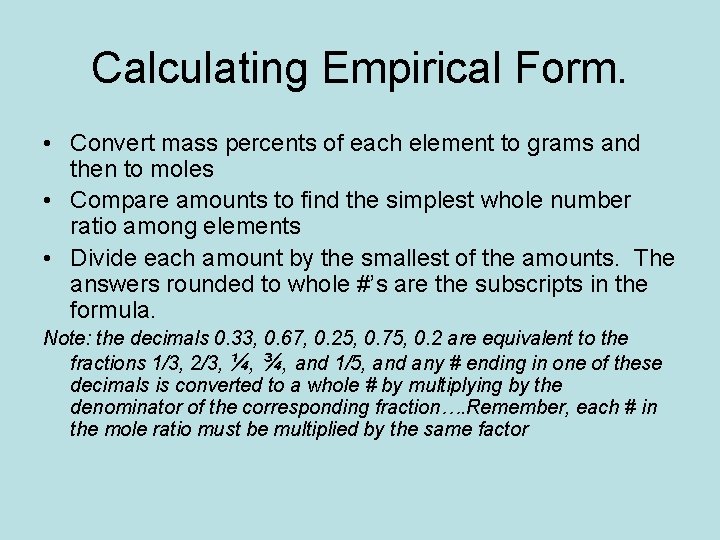
Calculating Empirical Form. • Convert mass percents of each element to grams and then to moles • Compare amounts to find the simplest whole number ratio among elements • Divide each amount by the smallest of the amounts. The answers rounded to whole #’s are the subscripts in the formula. Note: the decimals 0. 33, 0. 67, 0. 25, 0. 75, 0. 2 are equivalent to the fractions 1/3, 2/3, ¼, ¾, and 1/5, and any # ending in one of these decimals is converted to a whole # by multiplying by the denominator of the corresponding fraction…. Remember, each # in the mole ratio must be multiplied by the same factor
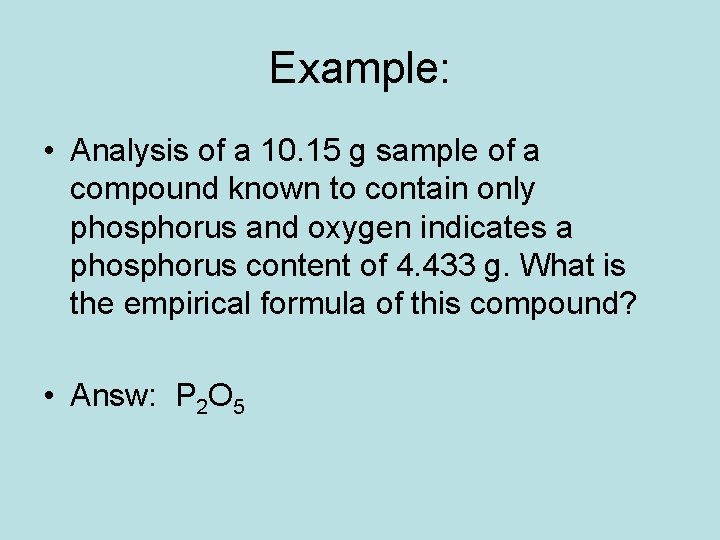
Example: • Analysis of a 10. 15 g sample of a compound known to contain only phosphorus and oxygen indicates a phosphorus content of 4. 433 g. What is the empirical formula of this compound? • Answ: P 2 O 5

More Examples: • One of the substances in a new alkaline battery is composed of 63% manganese and 37% oxygen. Determine the empirical formula. • Answ: Mn. O 2
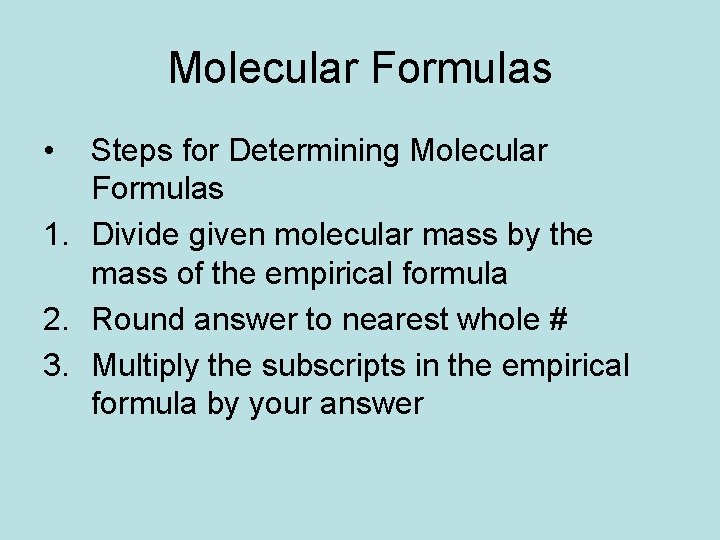
Molecular Formulas • Steps for Determining Molecular Formulas 1. Divide given molecular mass by the mass of the empirical formula 2. Round answer to nearest whole # 3. Multiply the subscripts in the empirical formula by your answer
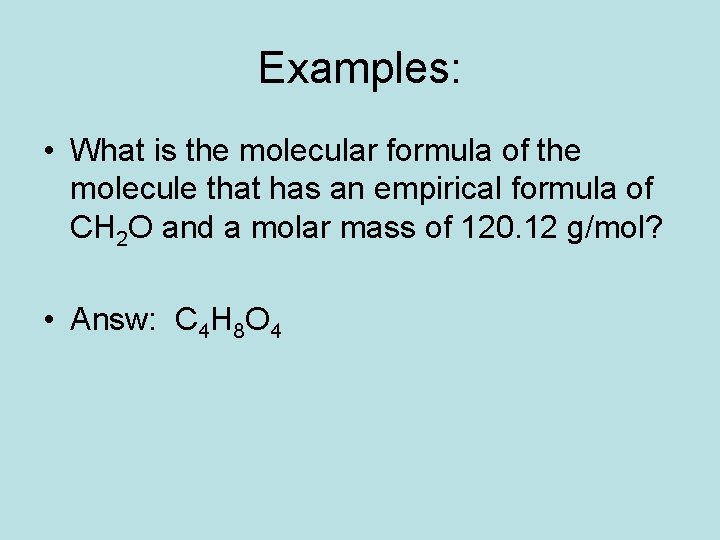
Examples: • What is the molecular formula of the molecule that has an empirical formula of CH 2 O and a molar mass of 120. 12 g/mol? • Answ: C 4 H 8 O 4
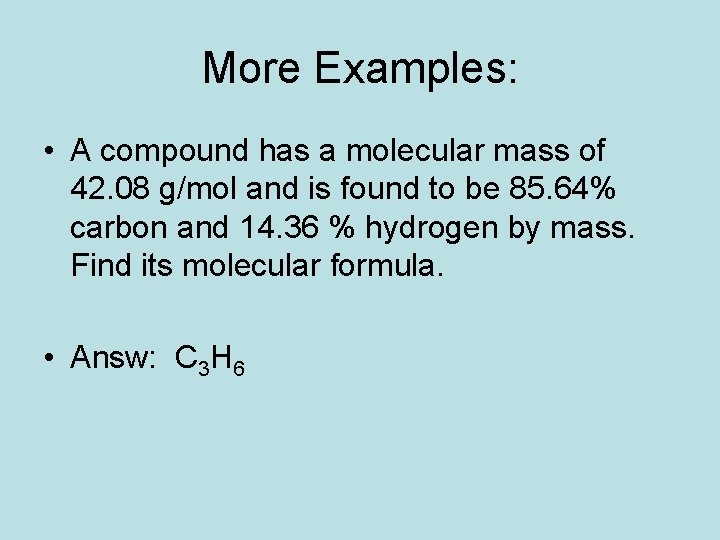
More Examples: • A compound has a molecular mass of 42. 08 g/mol and is found to be 85. 64% carbon and 14. 36 % hydrogen by mass. Find its molecular formula. • Answ: C 3 H 6
- Slides: 15