Definition States Change of State Types of Matter























- Slides: 60

Definition, States, Change of State, Types of Matter, and Properties and Changes Unit 1 B p. 2 -5.

MATTER, Definition, States, and Change of State At the conclusion of our time together, you should be able to: 1. Define matter 2. Define the various states of matter and draw an example of each state 3. Recognize that particle motion determines the state of matter

Matter: o Anything that has mass and takes up space Matter is made up of building blocks: atom – smallest unit of an element – a pure substance made of only one kind of atom. compound – made of two or more atoms that are chemically combined.

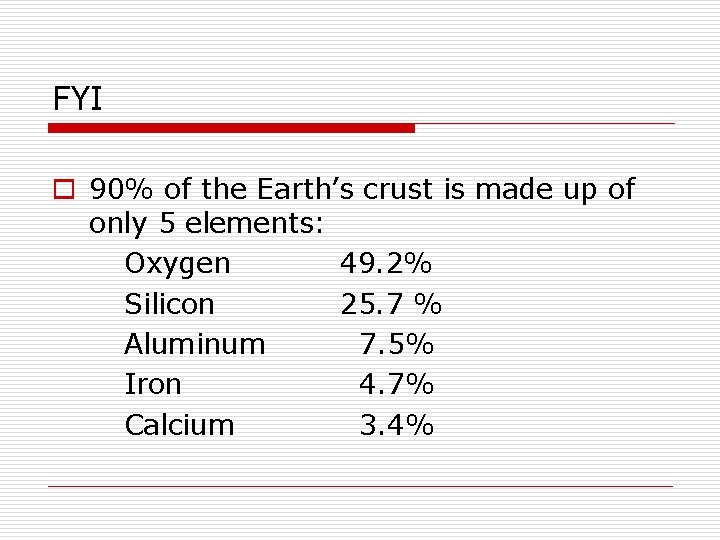
FYI o 90% of the Earth’s crust is made up of only 5 elements: Oxygen 49. 2% Silicon 25. 7 % Aluminum 7. 5% Iron 4. 7% Calcium 3. 4%

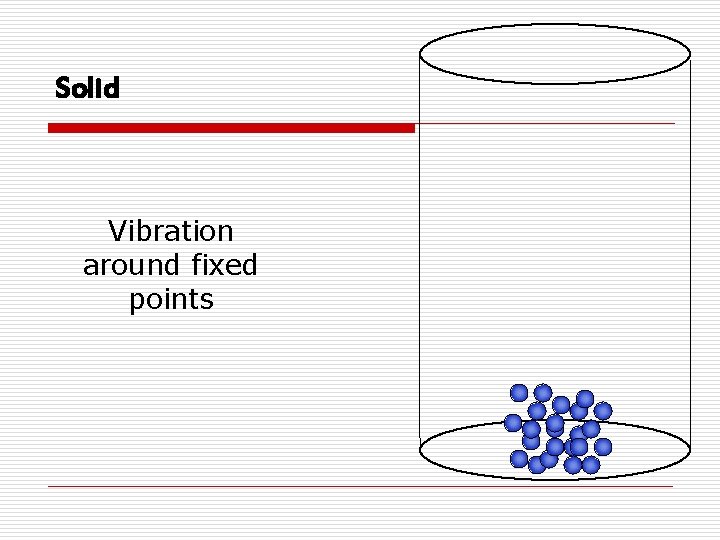
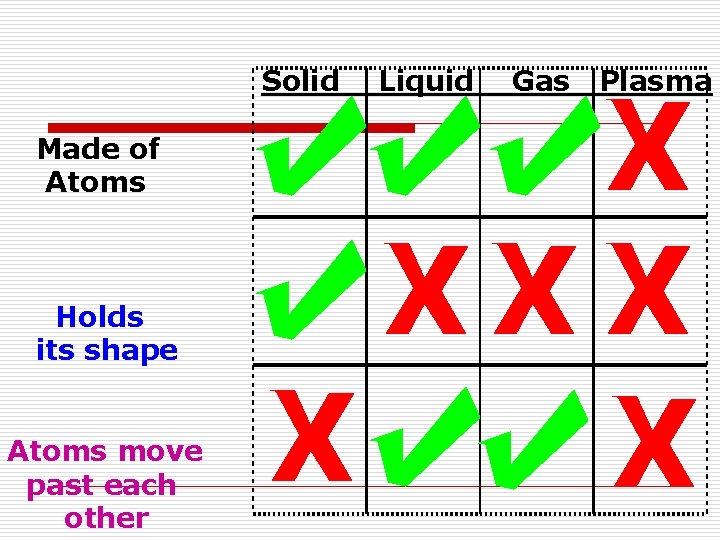
States of Matter o Solidn Definite volume and shape n Particles are tightly packed n Slight expansion when heated n Incompressible

Solid Vibration around fixed points

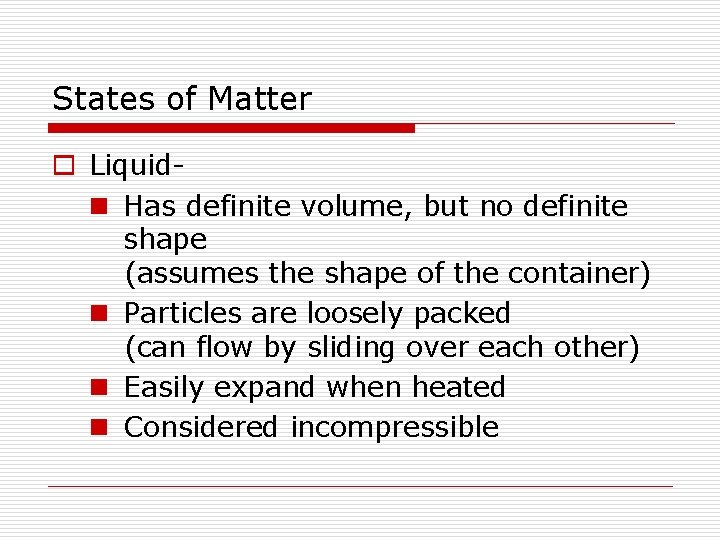
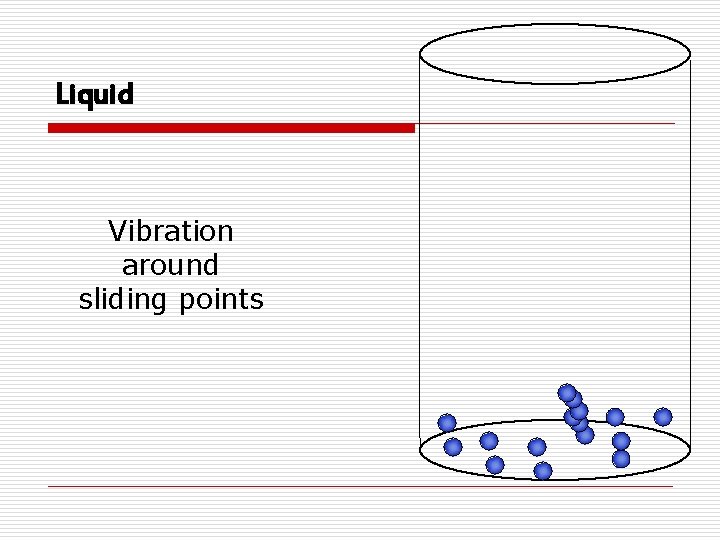
States of Matter o Liquidn Has definite volume, but no definite shape (assumes the shape of the container) n Particles are loosely packed (can flow by sliding over each other) n Easily expand when heated n Considered incompressible

Liquid Vibration around sliding points

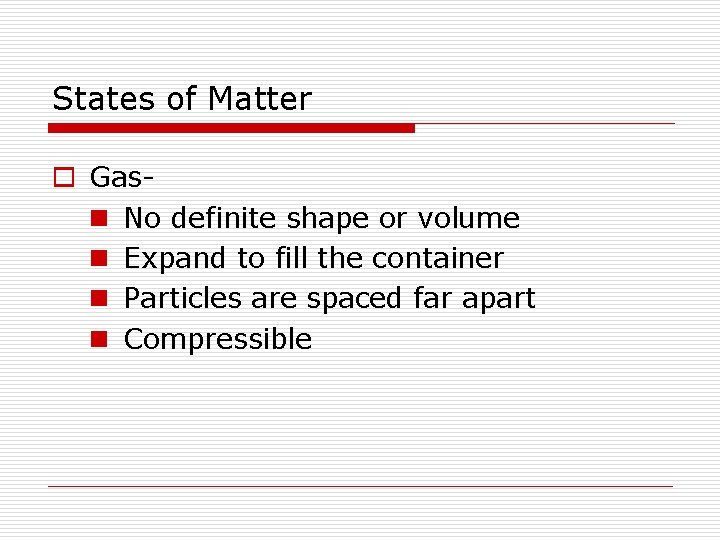
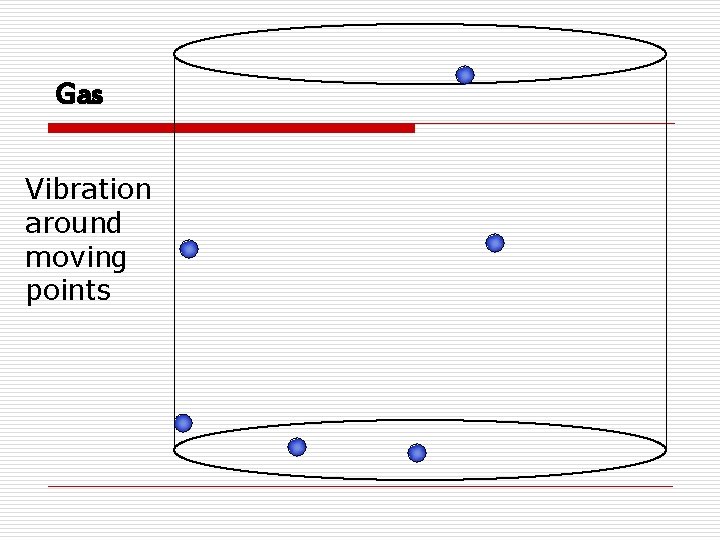
States of Matter o Gasn No definite shape or volume n Expand to fill the container n Particles are spaced far apart n Compressible

Gas Vibration around moving points

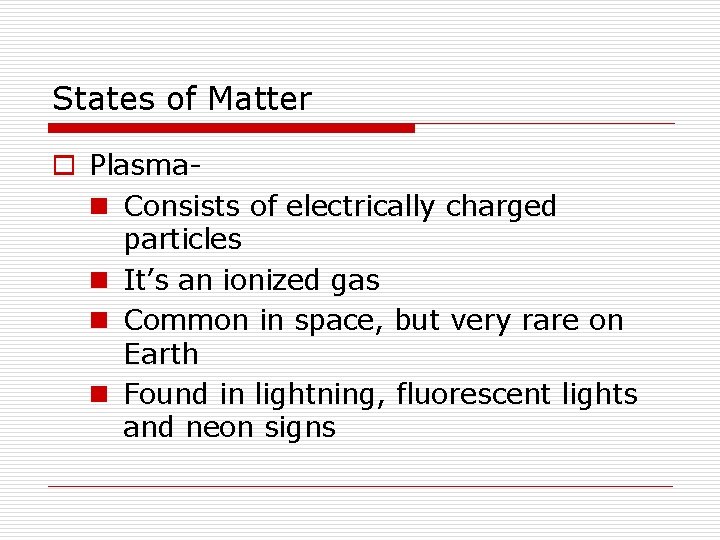
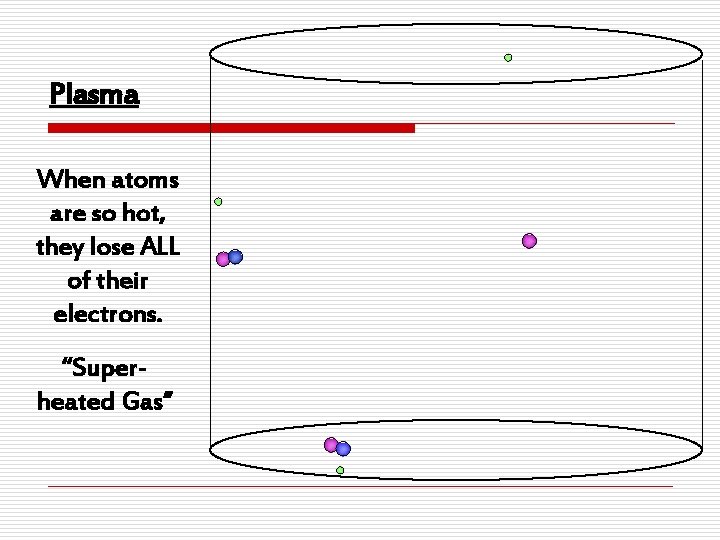
States of Matter o Plasman Consists of electrically charged particles n It’s an ionized gas n Common in space, but very rare on Earth n Found in lightning, fluorescent lights and neon signs

Plasma When atoms are so hot, they lose ALL of their electrons. “Superheated Gas”

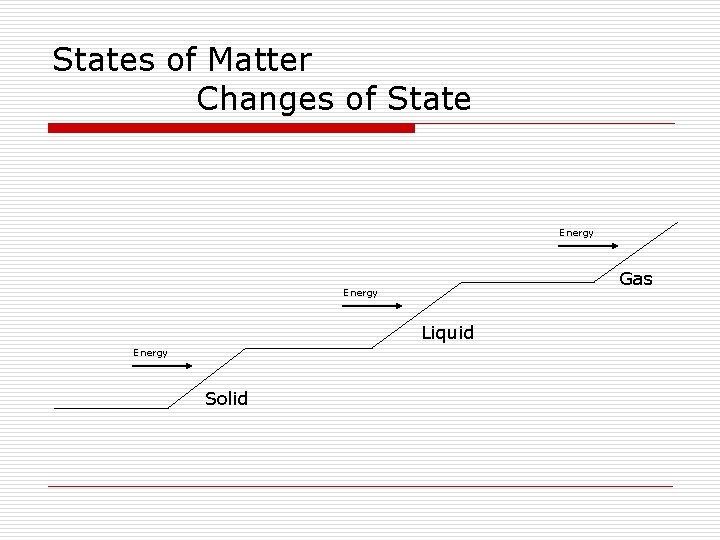
Energy Amounts in States of Matter o Solid- little energy, particles vibrate and rotate o Liquid- more energy, they move freely by sliding over each other o Gas- even more energy, move quickly o Plasma- most energy, move extremely fast

Solid Made of Atoms Holds its shape Atoms move past each other Liquid Gas Plasma

States of Matter Changes of State Energy Gas Energy Liquid Energy Solid

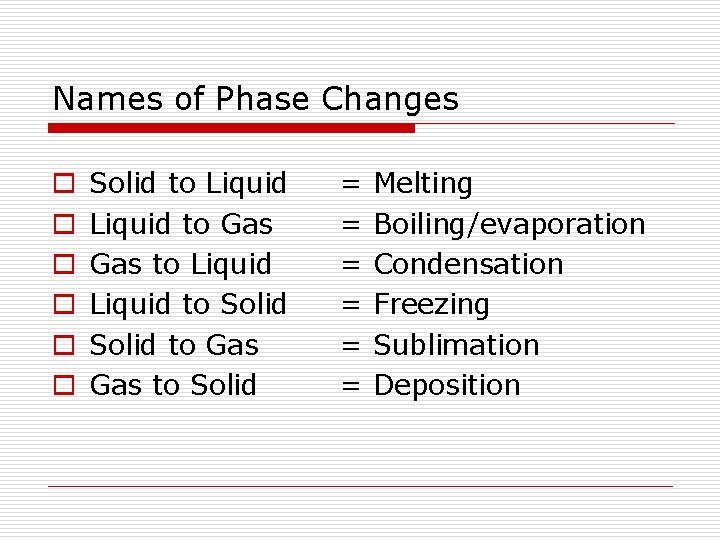
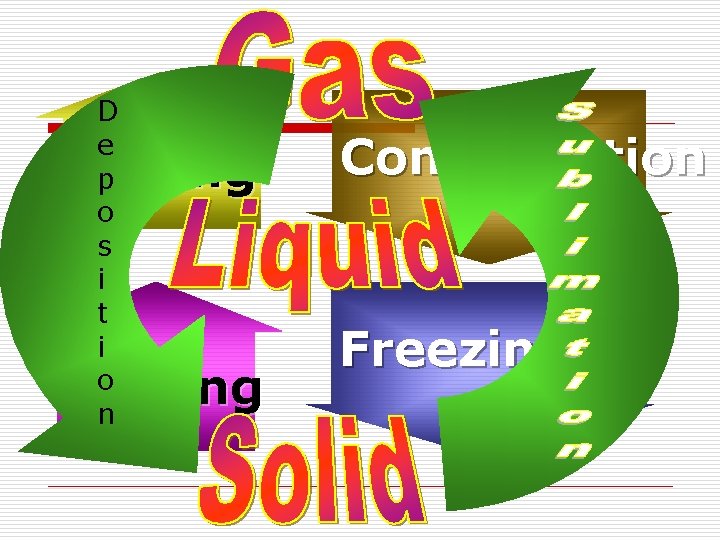
Names of Phase Changes o o o Solid to Liquid to Gas to Liquid to Solid to Gas to Solid = = = Melting Boiling/evaporation Condensation Freezing Sublimation Deposition

D e p o s i t i o n Boiling Melting Condensation Freezing

Sublimation When a solid turns directly into a gas. Dry ice is solid CO 2

Moisture that collects on the outside of a cold glass results from the process of… 1. evaporation. 2. condensation. 3. sublimation. 4. vaporization.

Matter, Classify Matter At the conclusion of our time together, you should be able to: 1. Classify a mixture of matter based on their physical and chemical properties 2. Characterize various types of matter

Types of Matter o Pure Substancen Matter with a fixed composition n It has distinct properties n Examples = elements compounds

Types of Matter o Mixturesn Most matter is a mixture n The composition is not fixed (changes from sample to sample) n Two Types – Homogeneous Heterogeneous

Homogeneous Mixtures o Composition is uniform throughout n Solutiono Particle size = 0. 01 – 1 nm o Doesn’t settle out upon standing o Can’t be separated by filtering o Doesn’t scatter light o Example = salt water

Homogeneous Mixtures n Colloido Particle size = 1 – 1000 nm o Doesn’t settle out upon standing o Can’t be separated by filtering o Scatters light (Tyndall Effect) o Examples = milk, gelatin, smoke

Heterogeneous Mixtures - Suspension o The sample varies in composition, properties and appearance o No uniformity n If a suspension: n Particle size is greater than 1000 nm n Particles settle out upon standing n Can be separated by filtration n Might scatter light n Examples = soil, trail mix, pond water

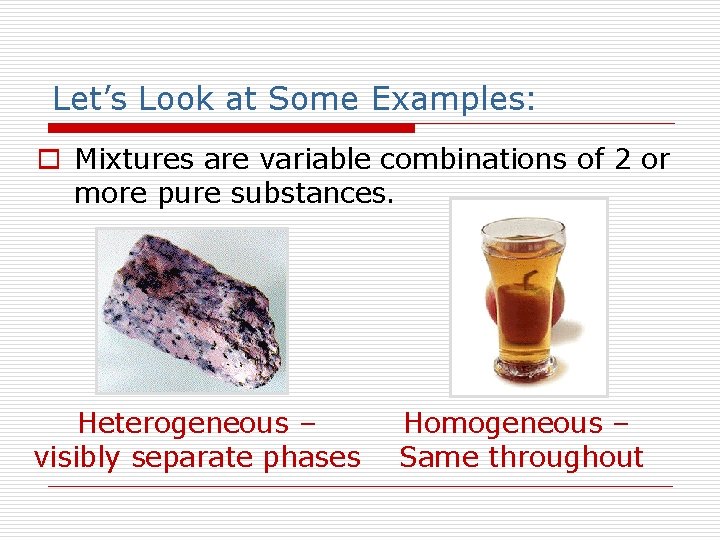
Let’s Look at Some Examples: o Mixtures are variable combinations of 2 or more pure substances. Heterogeneous – visibly separate phases Homogeneous – Same throughout

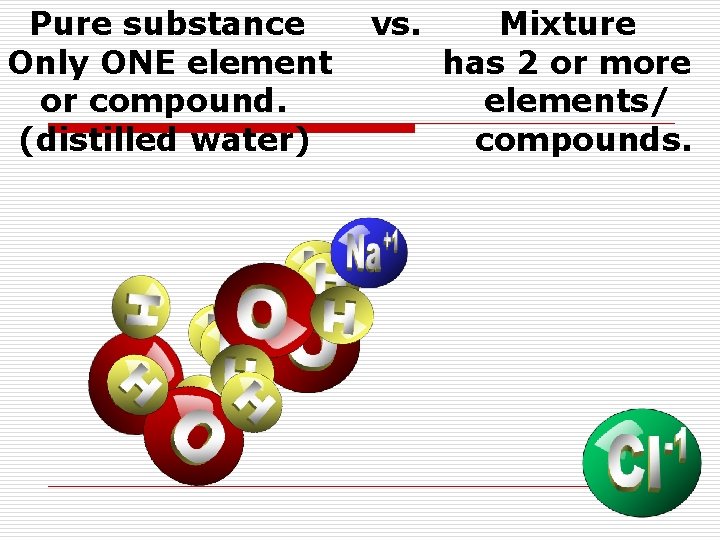
Pure substance Only ONE element or compound. (distilled water) vs. Mixture has 2 or more elements/ compounds.

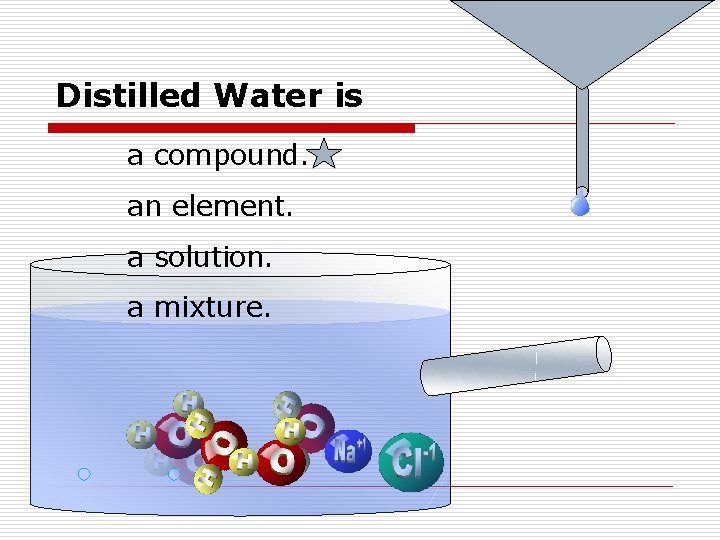
Distilled Water is a compound. an element. a solution. a mixture.

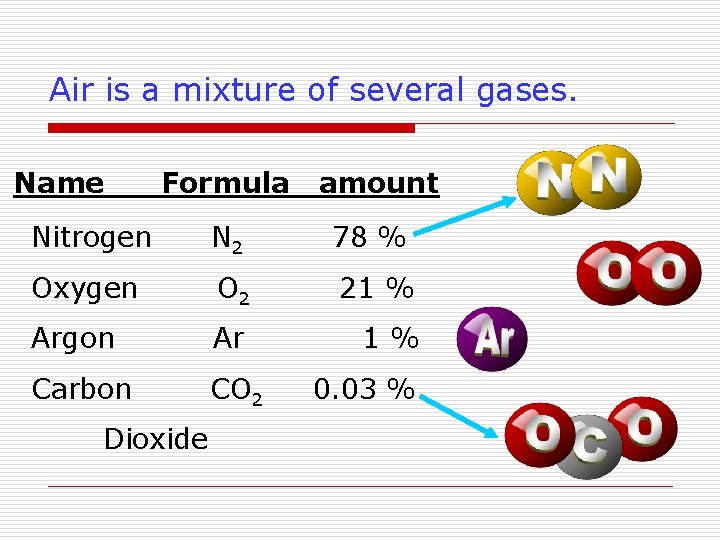
Air is a mixture of several gases. Name Formula amount Nitrogen N 2 78 % Oxygen O 2 21 % Argon Ar 1% Carbon CO 2 Dioxide 0. 03 %

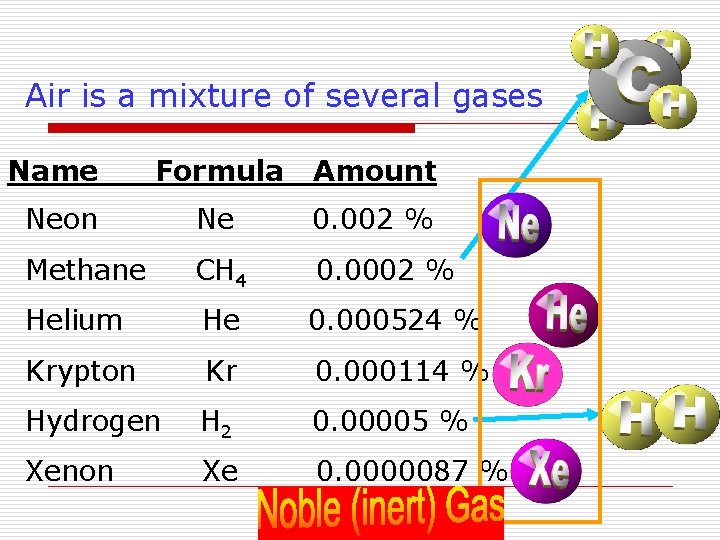
Air is a mixture of several gases Name Formula Amount Neon Ne 0. 002 % Methane CH 4 0. 0002 % Helium He 0. 000524 % Krypton Kr 0. 000114 % Hydrogen H 2 0. 00005 % Xenon Xe 0. 0000087 %

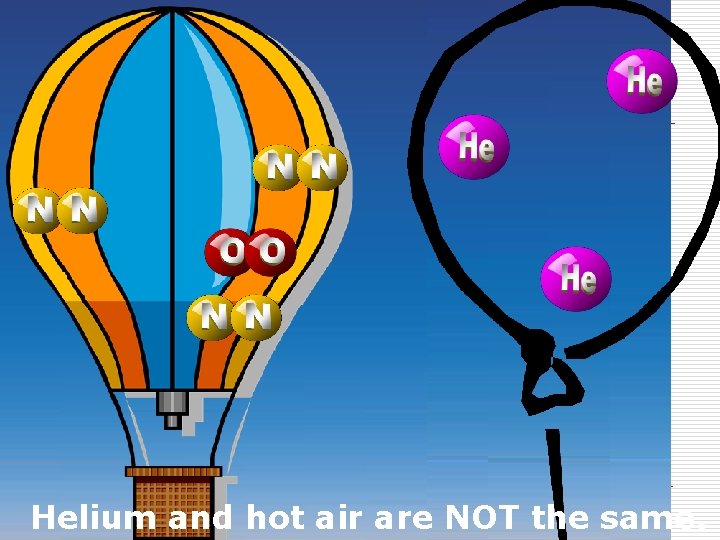
Atoms are NOT the same as molecules. Air and oxygen are NOT the same. Helium and hot air are NOT the same.

Helium and hot air are NOT the same.

What’s the MATTER Classify Matter Let’s Put it All Together in a Chart!!

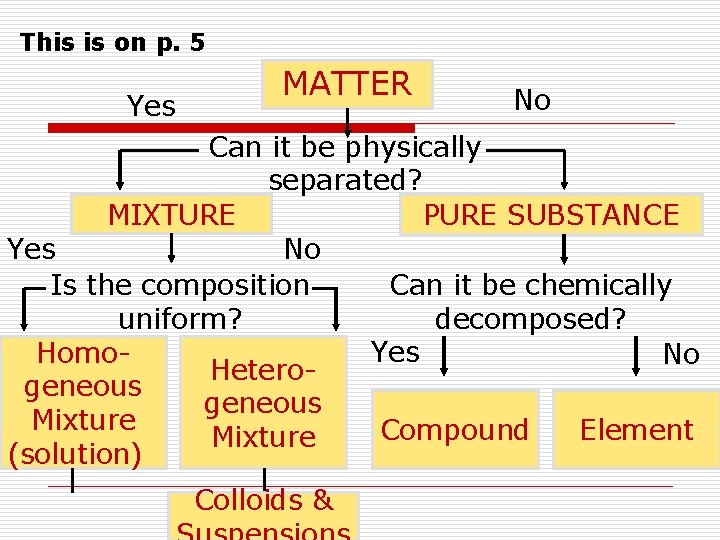
This is on p. 5 Yes MATTER No Can it be physically separated? MIXTURE PURE SUBSTANCE Yes No Can it be chemically Is the composition decomposed? uniform? Yes Homo. No Heterogeneous Mixture Compound Element Mixture (solution) Colloids &

Putting sand salt together makes a compound. an element. a mixture. a solution.

Pure Water is a compound. an element. a solution. a mixture.

Tap Water is a compound. an element. a solution. a mixture.

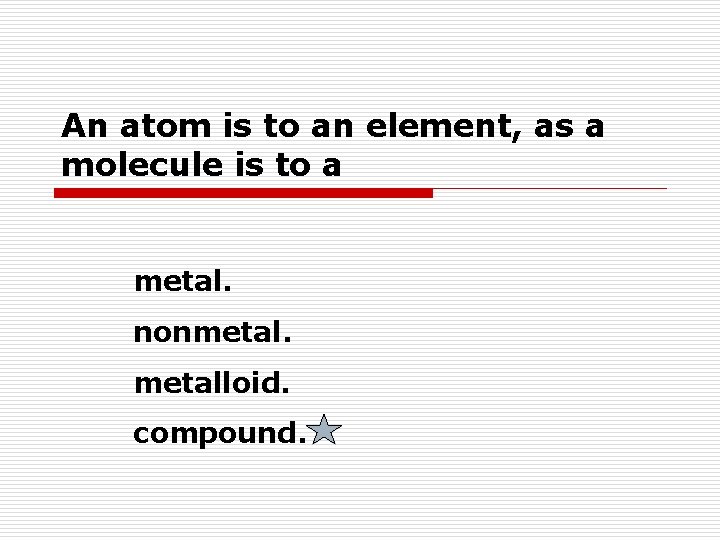
An atom is to an element, as a molecule is to a metal. nonmetalloid. compound.

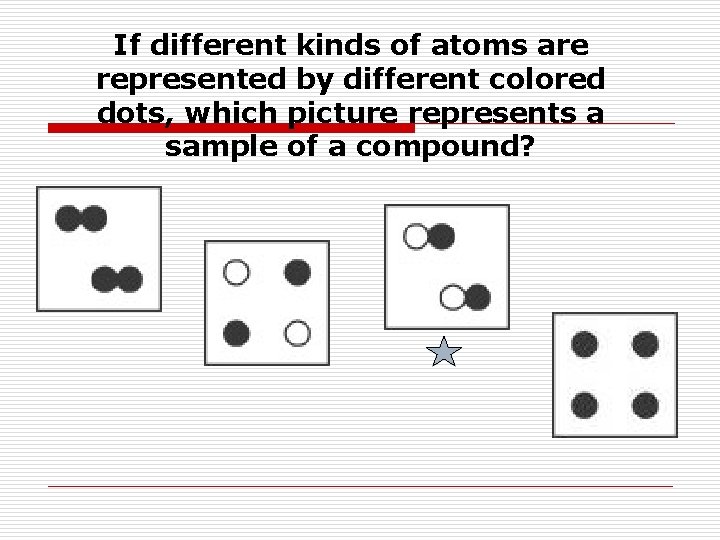
If different kinds of atoms are represented by different colored dots, which picture represents a sample of a compound?

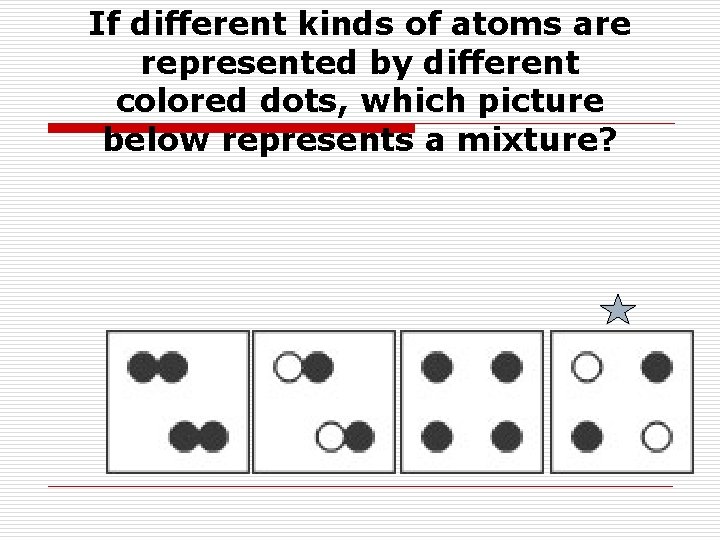
If different kinds of atoms are represented by different colored dots, which picture below represents a mixture?

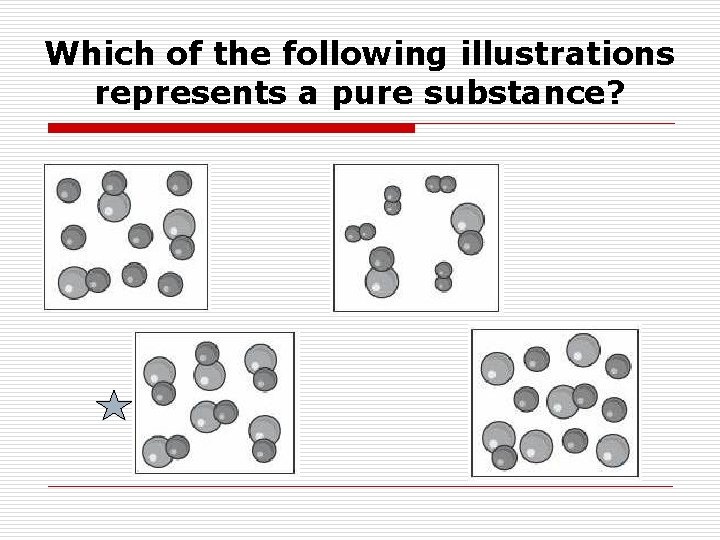
Which of the following illustrations represents a pure substance?

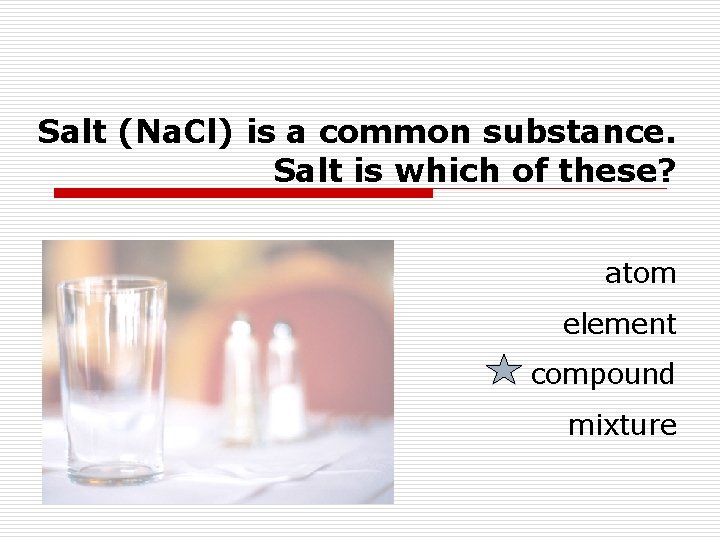
Salt (Na. Cl) is a common substance. Salt is which of these? atom element compound mixture

A chocolate chip cookie is an example of a _____, because _______. a. compound, the ingredients are chemically bonded. b. compound, it is the same throughout. c. mixture, you can separate out the chips. d. mixture, you cannot distinguish between the ingredients.

Which of the following is a compound? • oxygen • water • nitrogen • air

Physical Change Physical ØA change in matter from one form to another without changing its chemical properties Ø(most can be reversed) ØNo change in atoms/molecules

Physical Change o Examples = n Change in state or phase change n Dissolving n Compressing n Light emission/absorption n Electrons passing through metals

Physical Change More Examples = o boiling of a liquid o melting of a solid o dissolving a solid in a liquid to give a homogeneous mixture o making a solution

Properties o Every substance has a unique set of properties (characteristics that identify that substance) o Physical Propertiesn Properties that can be measured without changing the identity and composition of the substance

Physical Property Examplesn n n n Color Odor Density Melting Point Boiling Point Hardness Solubility

Chemical Change 2 1 Chemical A change in matter from one form to another by changing its composition (most cannot be reversed) Bonds are made / broken Chemical change or chemical reaction — transformation of one or more atoms or molecules into one or more different molecules.

Sure Signs of a Chemical Change “Chemists Get Practice Trying Labs” o Color Change o Gas Produced (not from boiling!) o Precipitate – a solid formed by mixing two liquids together o Temperature Change o Light

Chemical Properties o Properties that describe the way a substance may change to form other substances o Only observed when a chemical reaction takes place

Chemical Properties o Properties that describe the way a substance may change to form other substances o Only observed when a chemical reaction takes place

Chemical Property Examples o o o Combustible Reactive with water or acid Flammable Corrosive Decomposes in air

Remember the Law of Conservation of Mass o In a physical change or a chemical reaction, mass is neither created or destroyed (Antoine Lavoisier)

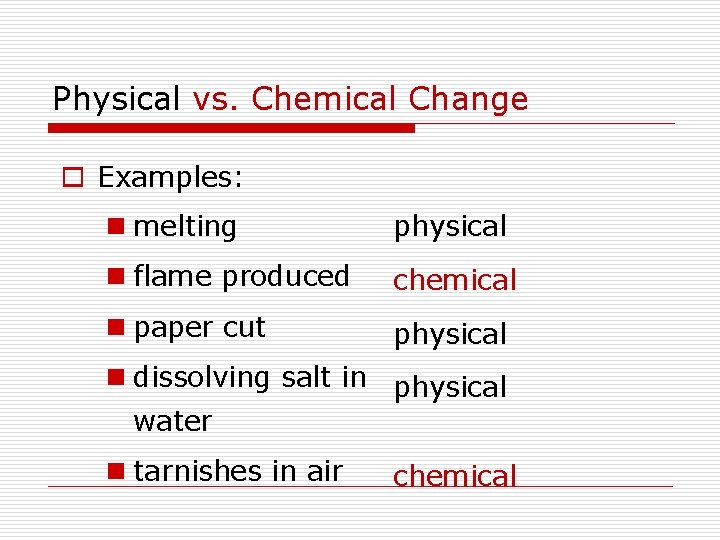
Physical vs. Chemical Change o Examples: n melting physical n flame produced chemical n paper cut physical n dissolving salt in physical water n tarnishes in air chemical

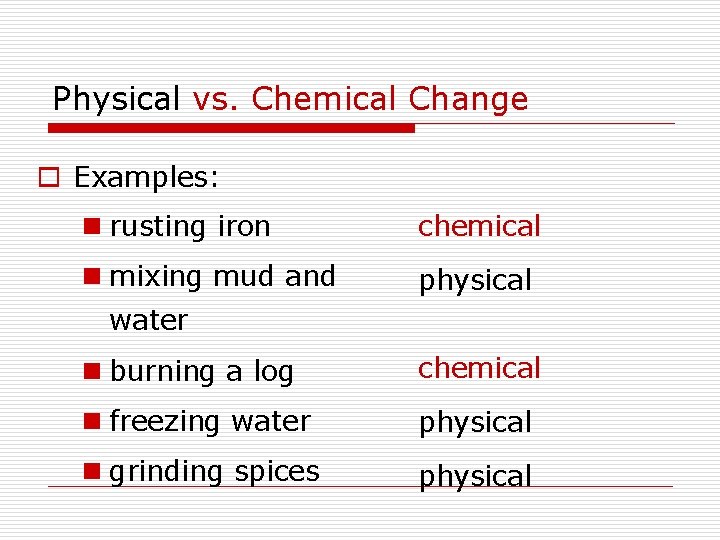
Physical vs. Chemical Change o Examples: n rusting iron chemical n mixing mud and physical water n burning a log chemical n freezing water physical n grinding spices physical

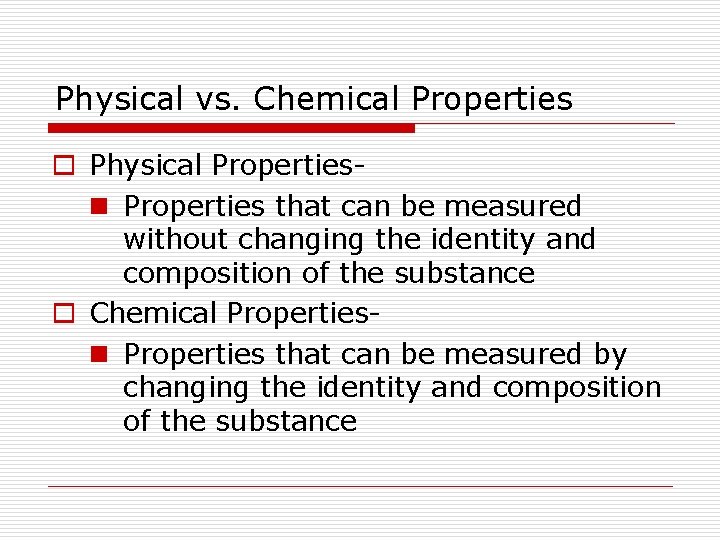
Physical vs. Chemical Properties o Physical Propertiesn Properties that can be measured without changing the identity and composition of the substance o Chemical Propertiesn Properties that can be measured by changing the identity and composition of the substance

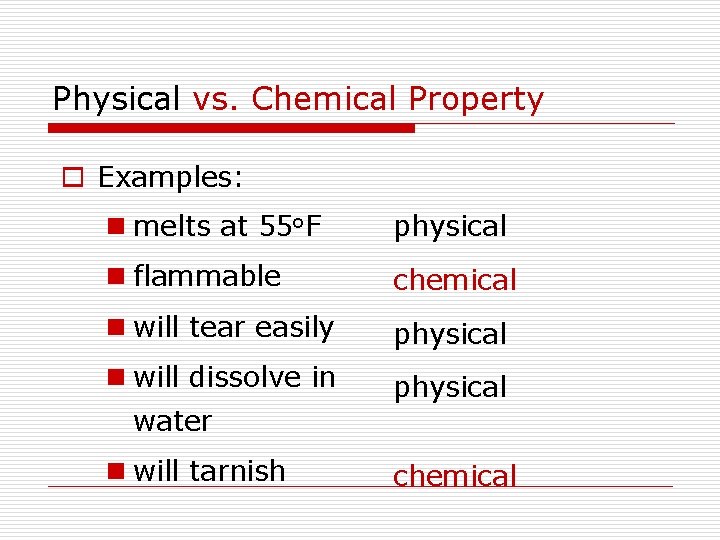
Physical vs. Chemical Property o Examples: n melts at 55 o. F physical n flammable chemical n will tear easily physical n will dissolve in physical water n will tarnish chemical

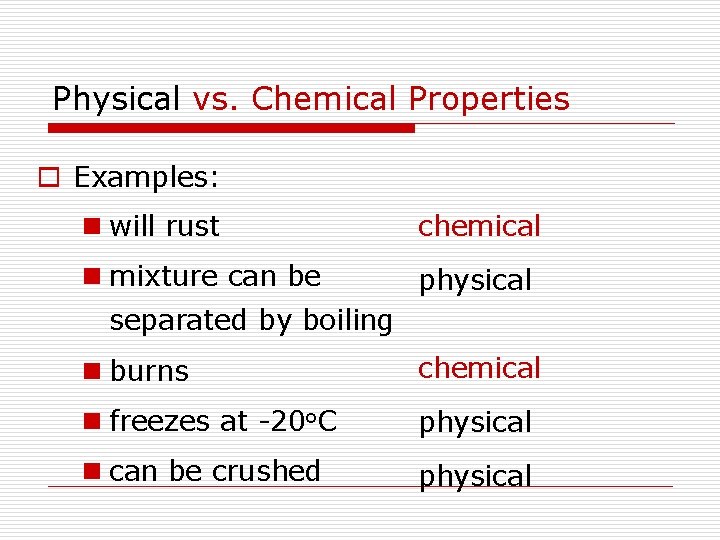
Physical vs. Chemical Properties o Examples: n will rust chemical n mixture can be physical separated by boiling n burns chemical n freezes at -20 o. C physical n can be crushed physical