Definition of terms Liquidus The line separating the



- Slides: 6

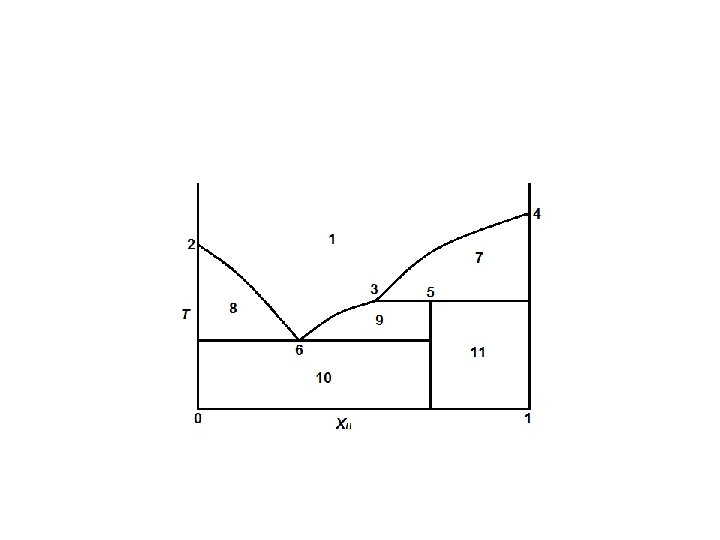
Definition of terms: Liquidus - The line separating the field of all liquid from that of liquid plus crystals. Solidus - The line separating the field of all solid from that of liquid plus crystals. Eutectic point - the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain constant until one of the phases disappears. A eutectic is an invariant point. Intermediate compound - A phase that has a composition intermediate between two other phases.

CONGRUENT = consistent; having exactly the same shape and size. Congruent melting - melting wherein a phase melts sharply to a liquid with the same composition as the solid. For example, Zn-Mg, Al-Mg, Au-Sn, etc. INCONGRUENT = unsuitable; not harmonious; out of place. Incongruent melting - melting wherein a phase melts to a liquid with a composition different from the solid and produces a solid of different composition to the original solid.

Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. A peritectic point is also an invariant point meaning?

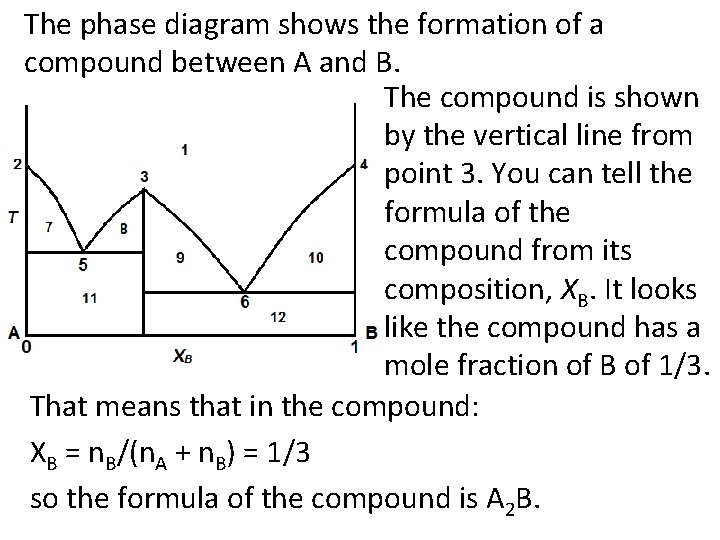
The phase diagram shows the formation of a compound between A and B. The compound is shown by the vertical line from point 3. You can tell the formula of the compound from its composition, XB. It looks like the compound has a mole fraction of B of 1/3. That means that in the compound: XB = n. B/(n. A + n. B) = 1/3 so the formula of the compound is A 2 B.

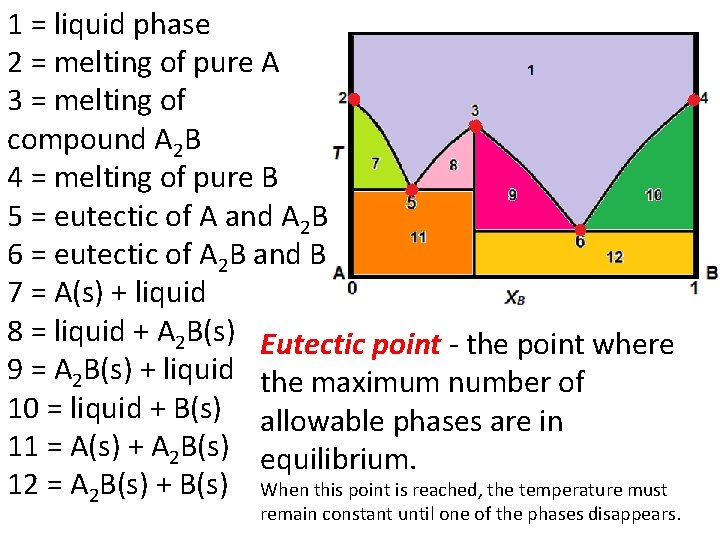
1 = liquid phase 2 = melting of pure A 3 = melting of compound A 2 B 4 = melting of pure B 5 = eutectic of A and A 2 B 6 = eutectic of A 2 B and B 7 = A(s) + liquid 8 = liquid + A 2 B(s) Eutectic point - the point where 9 = A 2 B(s) + liquid the maximum number of 10 = liquid + B(s) allowable phases are in 11 = A(s) + A 2 B(s) equilibrium. 12 = A 2 B(s) + B(s) When this point is reached, the temperature must remain constant until one of the phases disappears.
